(AMP)-Activated Protein Kinase: a New Target for Nutraceutical Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Enzymatic Synthesis of 1-Sinapoylglucose from Free
Enzymatic Synthesis of 1-Sinapoylglucose from Free Sinapic Acid and UDP-Glucose by a Cell-Free System from Raphanus sativus Seedlings Dieter Strack Botanisches Institut der Universität zu Köln, Gyrhofstr. 15, D-5000 Köln 41 Z. Naturforsch. 35 c, 204-208 (1980); received December 28, 1979 Raphanus, Brassicaceae, Phenylpropanoid Metabolism, Glucose Ester, Esterification. Protein extracts from seedlings of Raphanus sativus catalyze the transfer of the glucosyl moiety of UDP-glucose to the carboxyl group of phenolic acids. Enzymatic activity was determined spec- trophotometrically by measuring the increase in absorbance at 360 nm and/or by the aid of high performance liquid chromatography (HPLC). From 12 phenolic acids tested as acceptors, sinapic acid was by far the best substrate. The glu- cosyltransfer to sinapic acid has a pH optimum near 7 and requires as SH group for activity, p- Chloromercuribenzoate (PCMB) inhibits activity, which can be restored by the addition of di- thiothreitol (DTT). The formation of 1-sinapoylglucose was found to be a reversible reaction, sin ce the addition of UDP results in a breakdown of the ester. Introduction Materials and Methods Higher plants contain a large number of various Plant material and culture conditions are described hydroxycinnamoyl esters. Most of them might be elsewhere [10]. synthesized via hydroxycinnamoyl-CoA thiolesters as the activated reaction partners [1]. The widely Thin-layer chromatography occurring glucose esters [2], however, might be Phenolic acid derivatives were chromatographed exclusively synthesized from free hydroxycinnamic on microcrystalline cellulose (Avicel) in CAW, chlo acids and UDP-glucose [3, 4]. The formation of roform - acetic acid - water (3 : 2, water saturated) glucose esters of benzoic acids might follow the same and were detected under UV with and without NH3- mechanism [5], vapor. -

Brief Genetics Report Haplotype Structures and Large
Brief Genetics Report Haplotype Structures and Large-Scale Association Testing of the 5 AMP-Activated Protein Kinase Genes PRKAA2, PRKAB1, and PRKAB2 With Type 2 Diabetes Maria W. Sun,1,2 Jennifer Y. Lee,1,2 Paul I.W. de Bakker,1,2,3 Noe¨l P. Burtt,2 Peter Almgren,4 Lennart Råstam,5 Tiinamaija Tuomi,6 Daniel Gaudet,7 Mark J. Daly,2,8 Joel N. Hirschhorn,2,3,9 David Altshuler,1,2,3,8,10 Leif Groop,4,6 and Jose C. Florez1,2,8,10 AMP-activated protein kinase (AMPK) is a key molecular plasma glucose, or insulin sensitivity. Several nominal asso- regulator of cellular metabolism, and its activity is induced ciations of variants in PRKAA2 and PRKAB1 with BMI appear by both metformin and thiazolidinedione antidiabetic med- to be consistent with statistical noise. Diabetes 55:849–855, ications. It has therefore been proposed both as a putative 2006 agent in the pathophysiology of type 2 diabetes and as a valid target for therapeutic intervention. Thus, the genes that encode the various AMPK subunits are intriguing ype 2 diabetes arises from the complex interplay candidates for the inherited basis of type 2 diabetes. We therefore set out to test for the association of common of various pathophysiologic mechanisms involv- variants in the genes that encode three selected AMPK ing peripheral insulin resistance and relative subunits with type 2 diabetes and related phenotypes. Of Tinsulin insufficiency. The final expression of the the seven genes that encode AMPK isoforms, we initially diabetic phenotype is strongly influenced by inheritance; chose PRKAA2, PRKAB1, and PRKAB2 because of their however, with the exception of rare monogenic forms of higher prior probability of association with type 2 diabetes, diabetes, common type 2 diabetes is thought to have a based on previous reports of genetic linkage, functional polygenic architecture (1). -

GRAS Notice GRN 868 Agency Response Letter -Coffee Fruit Extract
U.S. FOOD & DRUG ADMINISTRATI ON CENTER FOR FOOD SAFETY &APPLIED NUTRITION Ashish Talati Amin Talati Wasserman, LLP 100 S. Wacker Drive Suite 2000 Chicago, IL 60606 Re: GRAS Notice No. GRN 000868 Dear Mr. Talati: The Food and Drug Administration (FDA, we) completed our evaluation of GRN 000868. We received the notice that you submitted on behalf of VDF FutureCeuticals, Inc. (VDF) on June 10, 2019, and filed it on August 19, 2019. VDF submitted an amendment to the notice on November 1, 2019, that clarified information related to the description of coffee fruit extract, batch compliance with specifications, dietary exposure, safety studies, and analytical method validation. The subject of the notice is coffee fruit extract for use as an ingredient and as an antioxidant in certain beverages, including flavored waters, coffee, tea, ready-to-mix (RTM) beverages, fruit juices, and vegetable juices/blends; nutritional and replacement milk products (pre-workout); clusters/bars; chocolate; candy; and chewing gum, at levels ranging from 20 mg to 300 mg/serving.1 This notice informs us of VDF ' sview. that these uses of coffee fruit extract are GRAS through scientific procedures. Our use of the term, "coffee fruit extract" in this letter is not our recommendation of that term as an appropriate common or usual name for declaring the substance in accordance with FDA's labeling requirements. Under 21 CFR 101.4, each ingredient must be declared by its common or usual name. In addition, 21 CFR 102.5 outlines general principles to use when establishing common or usual names for nonstandardized foods. -

Flavonols, Phenolic Acids and Antioxidant Activity of Some Red
Stark,A., A. Nyska,A. Zuckerman, and Z. MadarChanges in intestinal Vincken,J,-P., H. A. Schols, R. J. F. J. )omen,K. Beldnan, R. G. F. Visser, Tunicamuscularis following dietary liber feeding inrats. A morphometric andA. G. J. Voragen'.Pectin - the hairy thing. ln. Voragen, E,H. Schools, studyusing image analysis. Dig Dis Sci 40, 960-966 (1995). andB. t4sser(Eds.): Advances inPectin and Pectinase Research, 4Z-5g. Stark,A., A. Nyska, and Z. Madar. I\4etabolic and morphometric changes KlumerAcademic Publishers, Dortrecht, Niederlande (2003). insmall and large intestine inrats fed high-fiber diets. Toxicol pathol 24, Yoo,S.-H., M. Marshall, A.Hotchkiss, and H. G. Lee: Viscosimetric beha- 166-171(1996). vioursof high-methoxy andlow-methoxy pectin solutions. Food Hydro- Sugawa-Katayama,Y.,and A. ltuza.Morphological changes of smallin- coll20, 62-67 (2005). testinalmucosa inthe rats fed high pectin diets. Oyo Toshitsu Kagaku 3, Zhang,J., and J. fr. Lupton: Dielary fibers stimulate colonic cell prolifera- 335-341(1994) (in Japanisch). tionby different mechanisms atdifferent sites. Nutr Cancer ZZ.26l-276 Tamura,M., and H. SuzukiEffects of pectinon jejunaland ileal mor- (1 994) phologyand ultrastructurein adultmice. Ann Nutr Metab 41, 2SS-259 (1997) Flavonols,Phenolic Acids and Antioxidant Activity of Some Red Fruits LidijaJakobek#, Marijan Seruga, lvana Novak and MailinaMedvidovi6-Kosanovi6 DepartmentofApplied Chemistry and Ecology, Faculty of Food Technology,J J StrossmayerUniversity of0sijek, Kuhaceva 18, HR-31000 0sijek, Croatia Summary schwarzeund rote Johannisbeere) wurde mittelsHPlC-Methode be- Redfruits (blueberry, blackberry, chokeberry, strawberry, red raspberry, stimmt.Die Verbindungen wurden als Aglykon nach der Hydrolyse mit sweetcherry, sour cherry, elderberry, black currant and red currant) were 1,2mol dm 3 HCI analysiert. -

32-3099: PRKAB1 Recombinant Protein Description
9853 Pacific Heights Blvd. Suite D. San Diego, CA 92121, USA Tel: 858-263-4982 Email: [email protected] 32-3099: PRKAB1 Recombinant Protein Alternative Name : AMPK,HAMPKb,5'-AMP-activated protein kinase subunit beta-1,AMPK subunit beta-1,AMPKb,PRKAB1. Description Source : E.coli. PRKAB1 Human Recombinant produced in E.Coli is a single, non-glycosylated, polypeptide chain containing 293 amino acids (1-270 a.a.) and having a molecular mass of 32.8 kDa. The PRKAB1 is fused to a 23 amino acid His Tag at N- Terminus and purified by proprietary chromatographic techniques. 5'-AMP-activated protein kinase subunit beta-1 (PRKAB1) hinders protein, carbohydrate and lipid biosynthesis, in addition to cell growth and proliferation. AMPK is a heterotrimer comprised of an alpha catalytic subunit, and non-catalytic beta and gamma subunits. AMPK acts via direct phosphorylation of metabolic enzymes, and longer-term effects by phosphorylation of transcription regulators. PRKAB1 is a regulator of cellular polarity by remodeling the actin cytoskeleton; most likely by indirectly activating myosin. Beta non-catalytic subunit acts as a scaffold on which the AMPK complex compiles, through its C-terminus that joins alpha (PRKAA1 or PRKAA2) and gamma subunits (PRKAG1, PRKAG2 or PRKAG3). Product Info Amount : 5 µg Purification : Greater than 85% as determined by SDS-PAGE. The PRKAB1 protein solution (0.5mg/ml) contains 20mM Tris-HCl buffer (pH 8.0), 0.15M NaCl, Content : 10% glycerol and 1mM DTT. Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of Storage condition : time. -

Anti-Inflammatory, Antipyretic, and Analgesic Properties Of
molecules Article Anti-Inflammatory, Antipyretic, and Analgesic Properties of Potamogeton perfoliatus Extract: In Vitro and In Vivo Study Samar Rezq 1 , Mona F. Mahmoud 1,* , Assem M. El-Shazly 2 , Mohamed A. El Raey 3 and Mansour Sobeh 4,* 1 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt; [email protected] 2 Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt; [email protected] 3 National Research Centre, Department of Phytochemistry and Plant Systematics, Pharmaceutical Division, Dokki, Cairo 12622, Egypt; [email protected] 4 AgroBioSciences Research, Mohammed VI Polytechnic University, Lot 660–Hay MoulayRachid, Ben-Guerir 43150, Morocco * Correspondence: [email protected] (M.F.M.); [email protected] (M.S.) Abstract: Natural antioxidants, especially those of plant origins, have shown a plethora of biological activities with substantial economic value, as they can be extracted from agro-wastes and/or under exploited plant species. The perennial hydrophyte, Potamogeton perfoliatus, has been used traditionally to treat several health disorders; however, little is known about its biological and its medicinal effects. Here, we used an integrated in vitro and in vivo framework to examine the potential effect of P. perfoliatus on oxidative stress, nociception, inflammatory models, and brewer’s yeast-induced pyrexia in mice. Our results suggested a consistent in vitro inhibition of three enzymes, namely 5-lipoxygenase, cyclooxygenases 1 and 2 (COX-1 and COX-2), as well as a potent antioxidant effect. Citation: Rezq, S.; Mahmoud, M.F.; These results were confirmed in vivo where the studied extract attenuated carrageenan-induced paw El-Shazly, A.M.; El Raey, M.A.; Sobeh, edema, carrageenan-induced leukocyte migration into the peritoneal cavity by 25, 44 and 64% at 200, M. -

A New Computational Approach to Evaluating Systemic Gene–Gene Interactions in a Pathway Affected by Drug LY294002
processes Article A New Computational Approach to Evaluating Systemic Gene–Gene Interactions in a Pathway Affected by Drug LY294002 Shinuk Kim College of Kyedang General Education, Sangmyung University, Cheonan 31066, Korea; [email protected]; Tel.: +82-(41)-550-5452; Fax: +82-(41)-550-5439 Received: 14 August 2020; Accepted: 23 September 2020; Published: 1 October 2020 Abstract: In this study, we investigate how drugs systemically affect genes via pathways by integrating information from interactions between chemical compounds and molecular expression datasets, and from pathway information such as gene sets using mathematical models. First, we adopt drug-induced gene expression datasets; then, employ gene set enrichment analysis tools for selecting candidate enrichment pathways; and lastly, implement the inverse algorithm package for identifying gene–gene regulatory networks in a pathway. We tested LY294002-induced datasets of the MCF7 breast cancer cell lines, and found a CELL CYCLE pathway with 101 genes, ERBB signaling pathway consisting of 82 genes, and MTOR pathway consisting of 45 genes. We consider two interactions: quantity strength depending on number of interactions, and quality strength depending on weight of interaction as positive (+) and negative ( ) interactions. Our methods revealed ANAPC1-CDK6 ( 0.412) and − − ORC2L- CHEK1(0.951) for the CELL CYCLE pathway; INS-RPS6 ( 3.125) and PRKAA2-PRKAA2 − (+1.319) for the MTOR pathway; and CBLB-RPS6KB1 ( 0.141), RPS6KB1-CBLC (+0.238) for the ERBB − signaling pathway to be top quality interactions. Top quantity interactions discovered include 12; the CDC ( ,+) gene family for the CELL CYCLE pathway, 20; PIK3 ( ), 23; PIK3CG (+) for the MTOR − − pathway, 11; PAK ( ), 10; PIK3 (+) for the ERBB signaling pathway. -

Association Study of AMP-Activated Protein Kinase Subunit Genes In
European Journal of Endocrinology (2009) 161 405–409 ISSN 0804-4643 CLINICAL STUDY Association study of AMP-activated protein kinase subunit genes in polycystic ovary syndrome Kari Sproul1,2, Michelle R Jones3, Ricardo Azziz1,2,4 and Mark O Goodarzi1,3,4,5 1Department of Obstetrics and Gynecology, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA, 2Department of Obstetrics and Gynecology, the David Geffen School of Medicine at UCLA, Los Angeles, California 90095, USA, 3Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Cedars-Sinai Medical Center, 8700 Beverly Boulevard, Room B-131, Los Angeles, California 90048, USA, 4Department of Medicine, the David Geffen School of Medicine at UCLA, Los Angeles, California 90095, USA and 5Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA (Correspondence should be addressed to M O Goodarzi at Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Cedars-Sinai Medical Center; Email: [email protected]) Abstract Objective: To examine the genes for AMP-activated protein kinase (AMPK) subunits a2(PRKAA2) and g3(PRKAG3) as candidates for polycystic ovary syndrome (PCOS) and its component traits. Design and methods: A total of 287 white PCOS women were recruited from the reproductive endocrinology clinic at the University of Alabama at Birmingham and 187 white control subjects were recruited from the surrounding community. Seven PRKAA2 single nucleotide polymorphisms (SNPs) and four PRKAG3 SNPs were genotyped in PCOS cases and controls. Genotyping and association analysis were performed at Cedars-Sinai Medical Center. Results: Nominal associations of PRKAA2 variants with insulin-related traits and the PRKAG3 Pro71Ala variant with PCOS were not statistically significant after multiple testing correction. -
![AMPK Beta 1 (PRKAB1) Mouse Monoclonal Antibody [Clone ID: OTI3D10] Product Data](https://docslib.b-cdn.net/cover/2261/ampk-beta-1-prkab1-mouse-monoclonal-antibody-clone-id-oti3d10-product-data-622261.webp)
AMPK Beta 1 (PRKAB1) Mouse Monoclonal Antibody [Clone ID: OTI3D10] Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA813116S AMPK beta 1 (PRKAB1) Mouse Monoclonal Antibody [Clone ID: OTI3D10] Product data: Product Type: Primary Antibodies Clone Name: OTI3D10 Applications: WB Recommended Dilution: WB 1:500 Reactivity: Human, Mouse, Rat Host: Mouse Isotype: IgG1 Clonality: Monoclonal Immunogen: Human recombinant protein fragment corresponding to amino acids 2-270 of human PRKAB1 (NP_006244) produced in E.coli. Formulation: PBS (PH 7.3) containing 1% BSA, 50% glycerol and 0.02% sodium azide. Concentration: 1 mg/ml Purification: Purified from mouse ascites fluids or tissue culture supernatant by affinity chromatography (protein A/G) Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Predicted Protein Size: 30.2 kDa Gene Name: protein kinase AMP-activated non-catalytic subunit beta 1 Database Link: NP_006244 Entrez Gene 19079 MouseEntrez Gene 83803 RatEntrez Gene 5564 Human Q9Y478 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 AMPK beta 1 (PRKAB1) Mouse Monoclonal Antibody [Clone ID: OTI3D10] – TA813116S Background: Non-catalytic subunit of AMP-activated protein kinase (AMPK), an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism. In response to reduction of intracellular ATP levels, AMPK activates energy-producing pathways and inhibits energy- consuming processes: inhibits protein, carbohydrate and lipid biosynthesis, as well as cell growth and proliferation. -
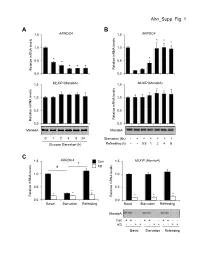
Ahn Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0
Ahn_Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0 * * 0.5 * 0.5 * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 1.5 MLXIP (MondoA) 1.5 MLXIP (MondoA) 1.0 1.0 0.5 0.5 Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 MondoA MondoA 0124824 Starvation (6h) -++++++ Glucose Starvation (h) Refeeding (h) --0.51248 C 1.5 ARRDC4 1.5 MLXIP (MondoA) † Con # KD 1.0 1.0 0.5 0.5 * * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative * * 0.0 0.0 BasalStarvation Refeeding BasalStarvation Refeeding MondoA Con + + - - + + - - + + - - KD - - + + - - + + - - + + BasalStarvation Refeeding Supplemental Figure 1. Glucose-mediated regulation of ARRDC4 is dependent on MondoA in human skeletal myotubes. (A) (top) ARRDC4 and MLXIP (MondoA) mRNA levels were determined by qRT-PCR in human skeletal myotubes following deprivation of glucose at the indicated time (n=4). (bottom) Representative Western blot analysis of MondoA demonstrating the effect of glucose deprivation. *p<0.05 vs. 0h. (B) (top) ARRDC4 and MLXIP (MondoA) expression in human myotubes following a 6h glucose removal and refeeding at the times indicated (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs Starvation 6h. (C) (top) Expression of ARRDC4 and MLXIP in human myotubes following deprivation and refeeding of glucose in the absence or presence of siRNA-mediated MondoA KD (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs siControl. # p<0.05. § p<0.05. The data represents mean ± SD. All statistical significance determined by one-way ANOVA with Tukey multiple comparison post-hoc test.