A Review of Phase Change Materials for Vehicle Component Thermal Buffering ⇑ Nicholas R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Inorganic Syntheses
INORGANIC SYNTHESES Volume 27 .................... ................ Board of Directors JOHN P. FACKLER, JR. Texas A&M University BODlE E. DOUGLAS University of Pittsburgh SMITH L. HOLT, JR. Oklahoma State Uniuersity JAY H. WORRELL University of South Florida RUSSELL N. GRIMES University of Virginia ROBERT J. ANGELIC1 Iowa State University Future Volumes 28 ROBERT J. ANGELIC1 Iowa State University 29 RUSSELL N. GRIMES University of Virginia 30 LEONARD V. INTERRANTE Rensselaer Polytechnic Institute 31 ALLEN H. COWLEY University of Texas, Austin 32 MARCETTA Y. DARENSBOURG Texas A&M University International Associates MARTIN A. BENNETT Australian National University, Canberra FAUSTO CALDERAZZO University of Pisa E. 0. FISCHER Technical University. Munich JACK LEWIS Cambridge University LAMBERTO MALATESTA University of Milan RENE POILBLANC University of Toulouse HERBERT W. ROESKY University of Gottingen F. G. A. STONE University of Bristol GEOFFREY WILKINSON Imperial College of Science and Technology. London AKlO YAMAMOTO Tokyo Institute 01 Technology. Yokohama Editor-in-Chief ALVIN P. GINSBERG INORGANIC SYNTHESES Volume 27 A Wiley-Interscience Publication JOHN WILEY & SONS New York Chichester Brisbane Toronto Singapore A NOTE TO THE READER This book has been electronically reproduced from digital idormation stored at John Wiley h Sons, Inc. We are phased that the use of this new technology will enable us to keep works of enduring scholarly value in print as long as there is a reasonable demand for them. The content of this book is identical to previous printings. Published by John Wiley & Sons, Inc. Copyright $? 1990 Inorganic Syntheses, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Section 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. -

Amélioration De La Maîtrise Des Ressources Françaises En Tungstène Improvement in Control of French Tungsten Resources BRGM
^ **' Amélioration de la maîtrise des ressources françaises en tungstène Improvement in control of french tungsten resources BRGM Rapport final M. SAVE M. PASTOR R 30346 Diffusion : MIN DAM 90 DG 1 ex page de garde, résumé, table mat., conclusions January 1990 LOG/D 1 ex non relié SGN/DIG 2 ex DS (M. Sustrac) 1 ex DAM/0P4 (M. Dumas) 1 ex DAM/MP (M. Snoep) 1 ex DAM/MIN : MM. Save 1 ex Morizot 1 ex Hau/Ollivier 1 ex chrono A.F.M.E. (M. Marcé) 6 ex CERMeP (M. Pastor) 1 ex IRCHA (Mme Presvot) 1 ex BUREAU DE RECHERCHES GÉOLOGIQUES ET MINIÈRES DIRECTION DES Activités minières Département Minéralurgie B.P. 6009 - 45060 ORLÉANS CEDEX 2 - France -Tél. : (33) 38.64.34.34 AMELIORATION DE LA MAITRISE DES RESSOURCES FRANÇAISES EN TUNGSTENE Responsable Scientifique : G. MORIZOT N* de contrat A.F.M.E. : 7.02.0035 Objet : Amélioration de la maîtrise de ressources françaises en tungstène Date de notification : 30/12/1987 Durée du contrat : 32 mois Montant du contrat : 1 600 000 F.F. H.T. Responsable A.F.M.E. : M. BEUTIN Service Industrie - Matières Premières RAPPORT CONFIDENTIEL BUREAU DE RECHERCHES GÉOLOGIQUES ET MINIÈRES DIRECTION DES ACTIVITÉS MINIERES Département Minêmlurqie B.P. G003 • 4E0H0 OKLEANS ŒDEX 2 - n.inc.* - îi'l.: (13) 23 ru IMJ.I R E S U M E Un programme général visant à améliorer la maîtrise des ressources françaises en tungstène a été entrepris par le B.R.G.M. et la Société EUROTUNGSTENE POUDRES (E.T.P.). Les études ont débuté le 1er octobre 1985 dans le cadre d'une première convention A.F.M.E. -

Geokniga-Introduction-Mineral-Exploration.Pdf
Introduction to Mineral Exploration Second Edition Edited by Charles J. Moon, Michael K.G. Whateley & Anthony M. Evans With contributions from William L. Barrett Timothy Bell Anthony M. Evans John Milsom Charles J. Moon Barry C. Scott Michael K.G. Whateley introduction to mineral exploration Introduction to Mineral Exploration Second Edition Edited by Charles J. Moon, Michael K.G. Whateley & Anthony M. Evans With contributions from William L. Barrett Timothy Bell Anthony M. Evans John Milsom Charles J. Moon Barry C. Scott Michael K.G. Whateley Copyright © 2006 by Charles J. Moon, Michael K.G. Whateley, and Anthony M. Evans BLACKWELL PUBLISHING 350 Main Street, Malden, MA 02148-5020, USA 9600 Garsington Road, Oxford OX4 2DQ, UK 550 Swanston Street, Carlton, Victoria 3053, Australia The rights of Charles J. Moon, Michael K.G. Whateley, and Anthony M. Evans to be identified as the Authors of this Work have been asserted in accordance with the UK Copyright, Designs, and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs, and Patents Act 1988, without the prior permission of the publisher. First edition published 1995 by Blackwell Publishing Ltd Second edition published 2006 1 2006 Library of Congress Cataloging-in-Publication Data Introduction to mineral exploration.–2nd ed. / edited by Charles J. Moon, Michael K.G. Whateley & Anthony M. Evans; with contributions from William L. Barrett . [et al.]. p. cm. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Cha Kuin Taiteit Van Et
CHA KUIN TAITEIT US009776210B2VAN ET (12 ) United States Patent ( 10 ) Patent No. : US 9 ,776 ,210 B2 Sarver et al. (45 ) Date of Patent: Oct . 3 , 2017 6 , 171, 383 B11 / 2001 Sakoske et al . ( 54 ) LASER ABSORBING COMPOUNDS 6 , 221 , 147 B1 4 /2001 Sakoske et al . 6 , 238, 847 B1 5 /2001 Axtell , III et al . (71 ) Applicant : Ferro Corporation , Mayfield Heights , 6 ,313 ,436 B1 11/ 2001 Harrison OH (US ) 6 , 416, 868 B1 7 /2002 Sullivan et al . 6 ,485 , 557 B1 11/ 2002 Swiler (72 ) Inventors : Joseph E . Sarver, Washington , PA 6 , 503 , 310 B1 1 / 2003 Sullivan (US ) ; Stephen Rozwood , Castle 6 , 503 , 316 B1 1 / 2003 Sakoske et al. 6 , 541 , 112 B1 4 / 2003 Swiler et al. Sharon , PA (US ) ; George E . Sakoske , 6 , 582 , 814 B2 6 / 2003 Swiler et al. Independence, OH (US ) ; Terry J . 6 , 680 , 121 B2 1 / 2004 Sakoske et al. Detrie , Washington , PA (US ) 6 , 852 , 948 B1 2 / 2005 Harrison 6 , 855 , 910 B2 2 / 2005 Harrison ( 73 ) Assignee : Ferro Corporation , Mayfield Heights , 6 , 924 , 077 B2 8 / 2005 Delp et al . 7 , 485 , 403 B2 2 / 2009 Khan OH (US ) 8 , 278 , 243 B2 10 /2012 Khan et al . 8 , 642 , 504 B2 2 / 2014 Campbell et al. ( * ) Notice : Subject to any disclaimer , the term of this 8 , 765 , 855 B2 7 / 2014 Thaker patent is extended or adjusted under 35 9 , 034 , 089 B2 5 /2015 Jarvis et al. U . S . C . 154 (b ) by 0 days . 9 , 321 , 130 B2 4 / 2016 Sarver et al. -

The Lithium Tungsten Bronzes
Scholars' Mine Masters Theses Student Theses and Dissertations 1949 The lithium tungsten bronzes Shun Sheng Hsu Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Metallurgy Commons Department: Recommended Citation Hsu, Shun Sheng, "The lithium tungsten bronzes" (1949). Masters Theses. 4869. https://scholarsmine.mst.edu/masters_theses/4869 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. MSM HISTORICAL COLLECTJO/f THE LITHIUM TUNGSTEN BRONZES By SHUN SHENG HSU --------- A THESIS submitted to the faculty of the SCHOOL OF MINES AND l'J1ET.ALLURGY OF THE UNIVERSITY OF MISSOURI in partial fulfillment of the work required for the Degree of MASTER OF SCIENCE IN METALLURGICAL ENGINEERING Rolla, Missouri 1949 MSM HISTORICAl COLLECTION Approved by______ ~~~·--~ __. __ (31,__ ~---~-~-~-~_,_·~--------------- Rese ch professor of Metallurgy ACKI~OWLEDGEMENT The author wishes to express his sincere apprec- iation to Dr. M. E. Straumanis, Research professor of lv'Iets.llurgy, Missouri School of Mines and Metallurgy, Rolls., Missouri for his many valuable suggestions which were g·iven during the course of this investigation; to Dr. A. w. Scblechten, Chairman of the Department of Metallurgy, Missouri School of Mines and Metall~rgy for the correction o:f the manuscript; and to Dr • . D. s. Eppelshe·imer, professor o:f Metallurgical Engineering, Missouri School o:f Mines and Metallurgy for the permis sion to use his x-ray machine :for diffraction work. -

HYSYS OLI Interface
HYSYS® 2004.2 OLI Interface Reference Guide Copyright October 2005 Copyright © 1981-2005 by Aspen Technology, Inc. All rights reserved. Aspen Accounting.21™, Aspen ACM Model Export, Aspen ACOL™, Aspen ACX™ Upgrade to ACOL™, Aspen Adsim®, Aspen Advisor™, Aspen Aerotran®, Aspen Alarm & Event™, Aspen APLE™, Aspen Apollo™, Aspen AtOMS™, Aspen Batch and Event Extractor, Aspen Batch Plus®, Aspen Batch.21™, Aspen Batch.21™ CBT, Aspen BatchCAD™, Aspen BatchSep™, Aspen Blend Model Library™, Aspen Blend™, Aspen BP Crude Oil Database, Aspen Calc CBT, Aspen Calc™, Aspen Capable-to-Promise®, Aspen CatRef®, Aspen Chromatography®, Aspen Cim-IO Core™, Aspen Cim-IO™ for @AGlance, Aspen Cim-IO™ for ABB 1180/ 1190 via DIU, Aspen Cim-IO™ for Bailey SemAPI, Aspen Cim-IO™ for DDE, Aspen Cim-IO™ for Eurotherm Gauge via DCP, Aspen Cim-IO™ for Fisher-Rosemount Chip, Aspen Cim-IO™ for Fisher-Rosemount RNI, Aspen Cim-IO™ for Foxboro FOXAPI, Aspen Cim-IO™ for G2, Aspen Cim-IO™ for GE FANUC via HCT, Aspen Cim-IO™ for Hitachi Ex Series, Aspen Cim-IO™ for Honeywell TDC 3000 via HTL/access, Aspen Cim-IO™ for Intellution Fix, Aspen Cim-IO™ for Measurex MCN, Aspen Cim-IO™ for Measurex ODX, Aspen Cim-IO™ for Moore Apacs via Nim (RNI), Aspen Cim-IO™ for OPC, Aspen Cim-IO™ for PI, Aspen Cim- IO™ for RSLinx, Aspen Cim-IO™ for SetCim/InfoPlus-X/InfoPlus.21, Aspen Cim-IO™ for Toshiba Tosdic, Aspen Cim-IO™ for ULMA 3D, Aspen Cim-IO™ for Westinghouse, Aspen Cim-IO™ for WonderWare InTouch, Aspen Cim-IO™ for Yokogawa ACG10S, Aspen Cim-IO™ for Yokogawa EW3, Aspen Collaborative Forecasting™, -
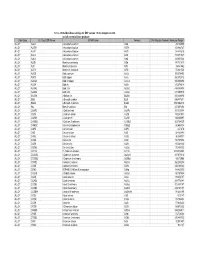
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -
Standard X-Ray Diffraction Powder Patterns
E^l Admin. NBS MONOGRAPH 25—SECTION 5 Refecii^M not to be ^ferlrom the library. Standard X-ray Diffraction Powder Patterns ^\ / U.S. DEPARTMENT OF COMMERCE S NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics— -Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Chemical Analysis of Alkali Metal Tungsten Bronzes Bruce Alan Raby Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Chemical analysis of alkali metal tungsten bronzes Bruce Alan Raby Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons Recommended Citation Raby, Bruce Alan, "Chemical analysis of alkali metal tungsten bronzes " (1963). Retrospective Theses and Dissertations. 2552. https://lib.dr.iastate.edu/rtd/2552 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 64—3888 microfilmed exactly as received RABY, Bruce Alan, 1930- CHEMICAL ANALYSIS OF ALKALI METAL TUNGSTEN BRONZES. Iowa State University of Science and Technology Ph.D., 1963 Chemistry, analytical University Microfilms, Inc., Ann Arbor, Michigan CHEMICAL ANALYSIS OF ALKALI METAL TUNGSTEN BRONZES by Bruce Alan Raby A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Analytical Chemistry Approved : Signature was redacted for privacy. Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1963 ii TABLE OF CONTENTS Page DEDICATION v I. THE ALKALI METAL TUNGSTEN BRONZES 1 A, Introduction to the Tungsten Bronzes 1 B. Prior Analytical Chemistry of the Alkali Metal Tungsten Bronzes 3 1. Decomposition and dissolution methods 3 2.