Standard X-Ray Diffraction Powder Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

Bismuth Antimony Telluride
ci al S ence Mahajan et al., J Material Sci Eng 2018, 7:4 ri s te & a E M n DOI: 10.4172/2169-0022.1000479 f g o i n l e a e n r r i n u g o Journal of Material Sciences & Engineering J ISSN: 2169-0022 Research Article Article OpenOpen Access Access Study and Characterization of Thermoelectric Material (TE) Bismuth Antimony Telluride Aniruddha Mahajan1*, Manik Deosarkar1 and Rajendra Panmand2 1Chemical Engineering Department, Vishwakarma Institute of Technology, Pune, India 2Centre for Materials Electronics and Technology (C-MET), Dr. Homi Bhabha Road, Pune, India Abstract Thermoelectric materials are used to convert the heat to electricity with no moving parts, in the present work an attempt has been made to prepare it for power generation function. Bismuth antimony telluride nanopowders were prepared by using mechanochemical method. Three different materials; Bismuth Telluride, (Bi0.75Sb0.25)2Te 3 and (Bi0.5Sb0.5)2Te 3 were synthesized. XRD and TEM analysis was carried out to confirm the results. The particle size of the material was determined by using FESEM analysis. The two alloys of Bismuth Telluride such prepared were converted in the pellet form using vacuum hydraulic pressure and their Seebeck coefficients were determined to test the material suitability for its use as a thermoelectric device. Their power factor measurement and Hall effect measurements were carried out at room temperature. Keywords: Bismuth telluride; Mechanochemical method; energy in one form into another. Use of TE solid materials Applications Nanoparticals; Seebeck coefficients in heat pump and refrigeration is well known [14] and it is now expanded such as cooled seats in luxury automobiles [15]. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Geochemical Modeling of Iron and Aluminum Precipitation During Mixing and Neutralization of Acid Mine Drainage
minerals Article Geochemical Modeling of Iron and Aluminum Precipitation during Mixing and Neutralization of Acid Mine Drainage Darrell Kirk Nordstrom U.S. Geological Survey, Boulder, CO 80303, USA; [email protected] Received: 21 May 2020; Accepted: 14 June 2020; Published: 17 June 2020 Abstract: Geochemical modeling of precipitation reactions in the complex matrix of acid mine drainage is fundamental to understanding natural attenuation, lime treatment, and treatment procedures that separate constituents for potential reuse or recycling. The three main dissolved constituents in acid mine drainage are iron, aluminum, and sulfate. During the neutralization of acid mine drainage (AMD) by mixing with clean tributaries or by titration with a base such as sodium hydroxide or slaked lime, Ca(OH)2, iron precipitates at pH values of 2–3 if oxidized and aluminum precipitates at pH values of 4–5 and both processes buffer the pH during precipitation. Mixing processes were simulated using the ion-association model in the PHREEQC code. The results are sensitive to the solubility product constant (Ksp) used for the precipitating phases. A field example with data on discharge and water composition of AMD before and after mixing along with massive precipitation of an aluminum phase is simulated and shows that there is an optimal Ksp to give the best fit to the measured data. Best fit is defined when the predicted water composition after mixing and precipitation matches most closely the measured water chemistry. Slight adjustment to the proportion of stream discharges does not give a better fit. Keywords: geochemical modeling; acid mine drainage; iron and aluminum precipitation; schwertmannite; basaluminite 1. -

CCXL1.- the Electrochemistry of Solutions in Acetone. Part I
View Article Online / Journal Homepage / Table of Contents for this issue 2 I38 ROSHDESTWENSKY AND LEWlS THE CCXL1.- The Electrochemistry of Solutions in Acetone. Part I. By ALEXANDERROSHDESTWENSEY and WILLIAM CUDMORE MCCULLAGHLEWIS. WHILSTa considerable amount of attention has been directed to the electrochemical behaviour of methyl and ethyl alcohols, rela- tively few measurements are recorded in the caw of solutions in acetone. The present paper contains the resulk of an investigation undertaken to supply to a certain extent this deficiency. The solutes employed were lithium nitrate and silver nitrate, the latter being examined in greater detail. Before giving our own meamrements, it is necessary to indicate briefly some of the results obtained by other observers which bear directly on the results obtained by us.* As regards the electrolysis of saturated silver nitrate solutions in acetone, Kahlenberg (J. physicd C‘Aem., 1900,4, 349) found that the metal was precipitated in a coherent form, but “the solution conducts so poorly ” that it was impossible to verify Faxaday’s law. In the same paper Kahlenberg gives some results of electromotive force measurements of Ag i AgNO, concentration cells in pyridine and acetonitrile respectively, with regard to which he concludes that the Nernst expressions are inapplicable. Kahlenberg asumes that the liquid/liquid potential difference is negligible, which, in the case of acetone at least, we shall show later is not the case. The specific conductivity of acetone itself has been meamred by Dutoit and Levier (J. Chim. phys., 1905, 3,435), the value obtained Published on 01 January 1911. Downloaded by Freie Universitaet Berlin 09/04/2018 17:53:03. -

Chemistry: the Molecular Nature of Matter and Change
REVISED CONFIRMING PAGES The Components of Matter 2.1 Elements, Compounds, and 2.5 The Atomic Theory Today 2.8 Formula, Name, and Mass of Mixtures: An Atomic Overview Structure of the Atom a Compound 2.2 The Observations That Led to Atomic Number, Mass Number, and Binary Ionic Compounds an Atomic View of Matter Atomic Symbol Compounds That Contain Polyatomic Mass Conservation Isotopes Ions Definite Composition Atomic Masses of the Elements Acid Names from Anion Names Multiple Proportions 2.6 Elements: A First Look at the Binary Covalent Compounds The Simplest Organic Compounds: Dalton’s Atomic Theory Periodic Table 2.3 Straight-Chain Alkanes Postulates of the Atomic Theory Organization of the Periodic Table Masses from a Chemical Formula How the Atomic Theory Explains Classifying the Elements Representing Molecules with a the Mass Laws Compounds: An Introduction 2.7 Formula and a Model to Bonding 2.4 The Observations That Led to Mixtures: Classification and the Nuclear Atom Model The Formation of Ionic Compounds 2.9 Separation Discovery of the Electron and The Formation of Covalent An Overview of the Components of Its Properties Compounds Matter Discovery of the Atomic Nucleus (a) (b) (right) ©Rudy Umans/Shutterstock IN THIS CHAPTER . We examine the properties and composition of matter on the macroscopic and atomic scales. By the end of this chapter, you should be able to • Relate the three types of matter—elements or elementary substances, compounds, and mixtures—to the simple chemical entities that they comprise—atoms, ions, and molecules; -

Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG
Central European Journal of Energetic Materials ISSN 1733-7178; e-ISSN 2353-1843 Copyright © 2018 Institute of Industrial Organic Chemistry, Poland Cent. Eur. J. Energ. Mater. 2018, 15(1): 115-130; DOI: 10.22211/cejem/78089 Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG Jing-yuan Zhang, Xue-yong Guo,* Qing-jie Jiao, Hong-lei Zhang, Hang Li State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China * E-mail: [email protected] Abstract: The thermal decomposition characteristics of CL-20, potassium perchlorate (KP), lithium perchlorate (LP), a CL-20/KP mixture, and a CL-20/LP mixture were studied using thermogravimetry-differential scanning calorimetry (TG-DSC). The DSC curves for KP exhibited three endothermic peaks and one exothermic peak. The first two endothermic peaks correspond to the rhombic- cubic transition and the fusion of KP, respectively, the third indicates the fusion of KCl, while the exothermic peak is attributed to the decomposition of KP. The DSC curves obtained from LP showed four endothermic peaks and one exothermic peak. The first two endothermic peaks indicate the loss of adsorbed water and water of crystallization, while the third and fourth are associated with the fusion of LP and LiCl, respectively; the exothermic peak is due to the decomposition of LP. The presence of KP had little effect on the thermal decomposition of CL-20 while the addition of LP increased the temperature at which CL-20 exhibits an exothermic peak. In addition, the thermal decomposition of LP appeared to be catalyzed by the presence of CL-20. -

Chemical Trends in Solid Alkali Pertechnetates † ‡ ‡ ‡ ‡ ‡ Jamie Weaver, , Chuck Z
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. Article pubs.acs.org/IC Chemical Trends in Solid Alkali Pertechnetates † ‡ ‡ ‡ ‡ ‡ Jamie Weaver, , Chuck Z. Soderquist, Nancy M. Washton, Andrew S. Lipton, Paul L. Gassman, § ∥ † † ‡ ⊥ Wayne W. Lukens, Albert A. Kruger, Nathalie A. Wall, and John S. McCloy*, , , † Department of Chemistry, Washington State University, Pullman, Washington 99164, United States ‡ Pacific Northwest National Laboratory, Richland, Washington 99352, United States § Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States ∥ U.S. Department of Energy (DOE), Office of River Protection, Richland, Washington 99352, United States ⊥ Materials Science and Engineering Program and School of Mechanical & Materials Engineering, Washington State University, Pullman, Washington 99164, United States *S Supporting Information ABSTRACT: Insight into the solid-state chemistry of pure technetium-99 (99Tc) oxides is required in the development of a robust immobilization and disposal system for nuclear waste stemming from the radiopharmaceutical industry, from the production of nuclear weapons, and from spent nuclear fuel. However, because of its radiotoxicity and the subsequent requirement of special facilities and handling procedures for research, only a few studies have been completed, many of which are over 20 years old. In this study, we report the synthesis of pure alkali pertechnetates (sodium, potassium, rubidium, and cesium) and analysis of these compounds by Raman spectroscopy, X-ray absorption spectroscopy (XANES and EXAFS), solid-state nuclear magnetic resonance (static and magic angle spinning), and neutron diffraction. The structures and spectral signatures of these compounds will aid in refining the understanding of 99Tc incorporation into and release from nuclear waste glasses. -
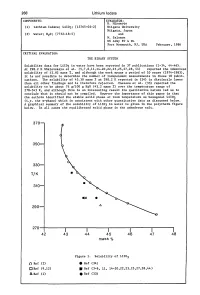
Evaluation of Aqueous Systems
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Inorganic Syntheses
INORGANIC SYNTHESES Volume 27 .................... ................ Board of Directors JOHN P. FACKLER, JR. Texas A&M University BODlE E. DOUGLAS University of Pittsburgh SMITH L. HOLT, JR. Oklahoma State Uniuersity JAY H. WORRELL University of South Florida RUSSELL N. GRIMES University of Virginia ROBERT J. ANGELIC1 Iowa State University Future Volumes 28 ROBERT J. ANGELIC1 Iowa State University 29 RUSSELL N. GRIMES University of Virginia 30 LEONARD V. INTERRANTE Rensselaer Polytechnic Institute 31 ALLEN H. COWLEY University of Texas, Austin 32 MARCETTA Y. DARENSBOURG Texas A&M University International Associates MARTIN A. BENNETT Australian National University, Canberra FAUSTO CALDERAZZO University of Pisa E. 0. FISCHER Technical University. Munich JACK LEWIS Cambridge University LAMBERTO MALATESTA University of Milan RENE POILBLANC University of Toulouse HERBERT W. ROESKY University of Gottingen F. G. A. STONE University of Bristol GEOFFREY WILKINSON Imperial College of Science and Technology. London AKlO YAMAMOTO Tokyo Institute 01 Technology. Yokohama Editor-in-Chief ALVIN P. GINSBERG INORGANIC SYNTHESES Volume 27 A Wiley-Interscience Publication JOHN WILEY & SONS New York Chichester Brisbane Toronto Singapore A NOTE TO THE READER This book has been electronically reproduced from digital idormation stored at John Wiley h Sons, Inc. We are phased that the use of this new technology will enable us to keep works of enduring scholarly value in print as long as there is a reasonable demand for them. The content of this book is identical to previous printings. Published by John Wiley & Sons, Inc. Copyright $? 1990 Inorganic Syntheses, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Section 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. -

Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843, 2846 or 2852 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

(12) United States Patent (10) Patent No.: US 6,358,747 B1 Condit Et Al
USOO6358747B1 (12) United States Patent (10) Patent No.: US 6,358,747 B1 Condit et al. (45) Date of Patent: Mar. 19, 2002 (54) METHOD AND APPARATUS FOR D. S. Gaibakyan etal, J. Anal. Chem. 1970, 25, 2056–2059.* QUANTIFYING MOLYBDATE IN BRINES A. M. Kiememeljet al, Anal. Chem. 1976, 48,575-578.* (75) Inventors: David A. Condit, Avon; Mark R. H. Llambias et al, Chem. Abstr. 1978, 88, abstract 53021f.* Jaworowski, Glastonbury; Xia Tang, West Hartford, all of CT (US) Lis et al., Journal of Alloys and Compounds 303-304, 132-136, 2000.* (73) ASSignee: sayier Corporation, Farmington, CT Water Analysis Handbood, HACH Company, Loveland, Colorado, “Molybdenum, Molybdate', pp. 636. (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 * cited by examiner U.S.C. 154(b) by 0 days. (21) Appl. No.: 09/324,376 Primary Examiner Arlen Soderquist y - - - 9 (22) Filed: Jun. 2, 1999 (57) ABSTRACT (51) Int. Cl. ................................................ G01N 33/20 Amethod and apparatus are provided for quantifying molyb (52) U.S. Cl. ............................. 436/83; 422/61; 436/73; date corrosion inhibitor concentrations in lithium halide 436/166 brines of absorption refrigeration Systems. This permits (58) Field of Search ............................ 436/73, 83, 166; monitoring and control of the inhibitor level. A reagent is 422/61 chosen for reacting with the molybdate in the brine to provide a readily identifiable characteristic color, the inten (56) References Cited sity of which is a function and measure of the molybdate U.S. PATENT DOCUMENTS concentration. The reagent is an acidified reducing agent a which reacts to provide a significant characteristic color 5,106,581.