Standard X-Ray Diffraction Powder Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paralaurionite Pbcl(OH) C 2001-2005 Mineral Data Publishing, Version 1
Paralaurionite PbCl(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals are thin to thick tabular k{100}, or elongated along [001], to 3 cm; {100} is usually dominant, but may show many other forms. Twinning: Almost all crystals are twinned by contact on {100}. Physical Properties: Cleavage: {001}, perfect. Tenacity: Flexible, due to twin gliding, but not elastic. Hardness = Soft. D(meas.) = 6.05–6.15 D(calc.) = 6.28 Optical Properties: Transparent to translucent. Color: Colorless, white, pale greenish, yellowish, yellow-orange, rarely violet; colorless in transmitted light. Luster: Subadamantine. Optical Class: Biaxial (–). Pleochroism: Noted in violet material. Orientation: Y = b; Z ∧ c =25◦. Dispersion: r< v,strong. Absorption: Y > X = Z. α = 2.05(1) β = 2.15(1) γ = 2.20(1) 2V(meas.) = Medium to large. Cell Data: Space Group: C2/m. a = 10.865(4) b = 4.006(2) c = 7.233(3) β = 117.24(4)◦ Z=4 X-ray Powder Pattern: Laurium, Greece. 5.14 (10), 3.21 (10), 2.51 (9), 2.98 (7), 3.49 (6), 2.44 (6), 2.01 (6) Chemistry: (1) (2) (3) Pb 78.1 77.75 79.80 O [3.6] 6.00 3.08 Cl 14.9 12.84 13.65 H2O 3.4 3.51 3.47 insol. 0.09 Total [100.0] 100.19 100.00 (1) Laurium, Greece. (2) Tiger, Arizona, USA. (3) PbCl(OH). Polymorphism & Series: Dimorphous with laurionite. Occurrence: A secondary mineral formed through alteration of lead-bearing slag by sea water or in hydrothermal polymetallic mineral deposits. -

Material Safety Data Sheet ______1
World Headquarters Page 1 Hach Company Date Printed 5/7/08 P.O.Box 389 MSDS No: M01321 Loveland, CO USA 80539 (970) 669-3050 MATERIAL SAFETY DATA SHEET _____________________________________________________________________________ 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Liquid Nitrate Ionic Strength Adjustor Buffer Catalog Number: 2488349 Hach Company Emergency Telephone Numbers: P.O.Box 389 (Medical and Transportation) Loveland, CO USA 80539 (303) 623-5716 24 Hour Service (970) 669-3050 (515)232-2533 8am - 4pm CST MSDS Number: M01321 Chemical Name: Not applicable CAS No.: Not applicable Chemical Formula: Not applicable Chemical Family: Not applicable Hazard: May cause irritation. Date of MSDS Preparation: Day: 15 Month: July Year: 2007 _____________________________________________________________________________ 2. COMPOSITION / INFORMATION ON INGREDIENTS Demineralized Water CAS No.: 7732-18-5 TSCA CAS Number: 7732-18-5 Percent Range: 90.0 - 100.0 Percent Range Units: weight / weight LD50: None reported LC50: None reported TLV: Not established PEL: Not established Hazard: No effects anticipated. Other components, each CAS No.: Not applicable TSCA CAS Number: Not applicable Percent Range: < 1.0 Percent Range Units: weight / volume LD50: Not applicable LC50: Not applicable TLV: Not established PEL: Not established Hazard: Any ingredient(s) of this product listed as "Other component(s)" is not considered a health hazard to the user of this product. Silver Sulfate CAS No.: 10294-26-5 TSCA CAS Number: 10294-26-5 World Headquarters Page 2 Hach Company Date Printed 5/7/08 P.O.Box 389 MSDS No: M01321 Loveland, CO USA 80539 (970) 669-3050 Percent Range: 0.1 - 1.0 Percent Range Units: weight / volume LD50: None reported LC50: None reported TLV: 0.01 mg/m³ (Ag) PEL: 0.01 mg/m³ (Ag) Hazard: Toxic properties unknown. -

Dielectric Behavior of Α-Ag2wo4 and Its Huge Dielectric Loss Tangent 1. Introduction
Materials Research. 2019; 22(4): e20190058 DOI: http://dx.doi.org/10.1590/1980-5373-MR-2019-0058 Dielectric Behavior of α-Ag2WO4 and its Huge Dielectric Loss Tangent Natalia Jacomacia* , Euripedes Silva Juniora , Fernando Modesto Borges de Oliveirab , Elson Longob , Maria Aparecida Zaghetea aInstituto de Química, Universidade Estadual Paulista (Unesp), 14800-060, Araraquara, SP, Brasil bDepartamento de Química, Universidade Federal de São Carlos (UFSCar), 13565-905, São Carlos, SP, Brasil Received: January 21, 2019; Revised: May 08, 2019; Accepted: June 29, 2019 The microwave-assisted hydrothermal method was used to obtain α-Ag2WO4. Rietveld refinement confirmed that α-Ag2WO4 is stable in the orthorhombic phase, without secondary phase. However, field- effect scanning electron microscope analysis showed that α-Ag2WO4 nanorods surfaces contain silver nanoparticles, confirmed by the X-ray photoelectron spectroscopy by the peak observed at 374.39 eV. In addition to metallic Ag, other Ag oxidation states were also observed on the surface. Hence, Ag (I) as Ag2O and Ag (I) as Ag2WO4 also were identified. DC measurements exhibited a high capacity of charge storage, nevertheless, with a large loss tangent (0.12 µC.cm-2.V-1) and no residual polarization for the voltage range between -100 V and +100 V. AC measurements at frequencies less than 275 Hz, revealed that ionic polarization is dominant, whereas at frequencies higher than 275 Hz, the electronic behavior predominates. The potential of electromagnetic energy conversion in thermal was observed from loss tangent analysis. Keywords: Silver tungstate nanorods, dielectric behavior, ionic and electronic polarization. 1. Introduction Among transition metal tungstates, silver tungstate (Ag2WO4) has attracted the attention of the scientific and technological Tungsten-silver materials are known to exhibit very good community because of its chemical stability, easy synthesis15, erosion resistance properties in low voltage. -

Chemistry: the Molecular Nature of Matter and Change
REVISED CONFIRMING PAGES The Components of Matter 2.1 Elements, Compounds, and 2.5 The Atomic Theory Today 2.8 Formula, Name, and Mass of Mixtures: An Atomic Overview Structure of the Atom a Compound 2.2 The Observations That Led to Atomic Number, Mass Number, and Binary Ionic Compounds an Atomic View of Matter Atomic Symbol Compounds That Contain Polyatomic Mass Conservation Isotopes Ions Definite Composition Atomic Masses of the Elements Acid Names from Anion Names Multiple Proportions 2.6 Elements: A First Look at the Binary Covalent Compounds The Simplest Organic Compounds: Dalton’s Atomic Theory Periodic Table 2.3 Straight-Chain Alkanes Postulates of the Atomic Theory Organization of the Periodic Table Masses from a Chemical Formula How the Atomic Theory Explains Classifying the Elements Representing Molecules with a the Mass Laws Compounds: An Introduction 2.7 Formula and a Model to Bonding 2.4 The Observations That Led to Mixtures: Classification and the Nuclear Atom Model The Formation of Ionic Compounds 2.9 Separation Discovery of the Electron and The Formation of Covalent An Overview of the Components of Its Properties Compounds Matter Discovery of the Atomic Nucleus (a) (b) (right) ©Rudy Umans/Shutterstock IN THIS CHAPTER . We examine the properties and composition of matter on the macroscopic and atomic scales. By the end of this chapter, you should be able to • Relate the three types of matter—elements or elementary substances, compounds, and mixtures—to the simple chemical entities that they comprise—atoms, ions, and molecules; -

Iodination of 3,5-Dichloroanisole Using Silver Salts
IODINATION OF 3,5-DICHLOROANISOLE USING SILVER SALTS By Alex L Fan A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements of the Sally McDonnell Barksdale Honors College. Oxford May 2017 Approved by ____________________________ Advisor: Professor Daniell Mattern ____________________________ Reader: Professor Davita Watkins ____________________________ Reader: Professor Nathan Hammer © 2017 Alex L Fan ALL RIGHTS RESERVED ii ABSTRACT Iodination of 3,5-dichloroanisole using silver salts (under the direction of Daniell Mattern) A paper published by Joshi et al. showed lower than expected yields in mono- and di-iodination of 3,5-dichloroanisole in dichloromethane using silver salts. One possibility for this could be that the starting material was being tri-iodinated and precipitating out of the solution. We used similar reaction conditions in attempts to prepare and isolate tri- iodinated product, but attempts of recrystallization from ethanol, DMSO, and 2- methoxyethanol showed no signs of it. Removal of silver iodide from the solids formed during the reaction using aqueous potassium iodide resulted in complete dissolution of the entire crude product, suggesting no organic product as well. This reaction was then combined with the concept of a solvent-less reaction based on a paper published by Bose et al. involving electrophilic aromatic halogenation using a ball milling machine. Variation of reagent equivalents, heat, and time have shown various percent compositions of the products 3,5-dichloro-2-iodoanisole -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Calcium Sulfate Precipitation Throughout Its Phase Diagram
Chapter 12 Calcium Sulfate Precipitation Throughout Its Phase Diagram Alexander E.S. Van Driessche, Tomasz M. Stawski, Liane G. Benning, and Matthias Kellermeier 12.1 Introduction Over the past decade, significant progress has been made in our understanding of crystallization phenomena. In particular, a number of precursor and intermediate species, both solutes and solids, and either stable or metastable (or even unstable), have been identified. Their existence extends the simplified picture of classical nucleation and growth theories toward much more complex pathways. These newly found species include various solute clusters, liquid-like phases, amorphous particles, and metastable (often nanosized) crystalline polymorphs, all of which may exist for different periods and may convert into one another depending on the chosen conditions (see, for instance, De Yoreo et al. 2017, Chap. 1; Wolf and Gower 2017, Chap. 3; Rodriguez-Blanco et al. 2017, Chap. 5; Birkedal 2017, Chap. 10; Reichel and Faivre 2017, Chap. 14, and references therein). Quite naturally, most A.E.S. Van Driessche Driessche () University Grenoble Alpes, CNRS, ISTerre, F-38000 Grenoble, France e-mail: [email protected] T.M. Stawski German Research Centre for Geosciences, GFZ, D-14473 Potsdam, Germany School of Earth and Environment, University of Leeds, LS2 9JT Leeds, UK L.G. Benning German Research Center for Geosciences, GFZ, Interface Geochemistry Section, 14473 Potsdam, Germany Department of Earth Sciences, Free University of Berlin, 12249 Berlin, Germany School of Earth and Environment, University of Leeds, Leeds LS2 9JT, UK M. Kellermeier () Material Physics, BASF SE, Carl-Bosch-Str. 38, D-67056 Ludwigshafen, Germany e-mail: [email protected] © Springer International Publishing Switzerland 2017 227 A.E.S. -

Oxygenic Bismuth Minerals in the NE Part of the Karkonosze Pluton (West Sudetes, SW Poland)
Acta Geologica Polonica, Vol. 68 (2018), No. 4, pp. 537–554 DOI: 10.1515/agp-2018-0016 Oxygenic bismuth minerals in the NE part of the Karkonosze pluton (West Sudetes, SW Poland) ANDRZEJ KOZŁOWSKI and WITOLD MATYSZCZAK* Faculty of Geology, University of Warsaw, Żwirki i Wigury 93, PL-02-089 Warszawa, Poland. *E-mail: [email protected] ABSTRACT: Kozłowski, A. and Matyszczak, W. 2018. Oxygenic bismuth minerals in the NE part of the Karkonosze pluton (West Sudetes, SW Poland). Acta Geologica Polonica, 68 (4), 537–554. Warszawa. The study presents fifteen oxygen-bearing secondary minerals of bismuth from the north-eastern part of the Variscan Karkonosze granitoid pluton in the northern zone of the Bohemian massif. The minerals were inves- tigated by optical, electron microprobe, classic chemical, XRD, IR absorption and fluid inclusion methods. The late, very low temperature epithermal solutions most probably caused formation of sillénite, kusachiite, bismoclite, bismutite, beyerite, kettnerite, pucherite, schumacherite, namibite and eulytite. Solutions dominated by supergene (meteoric) waters were the parents for bismite, russellite, koechlinite, ximengite and walpurgite. The paper also contains information on early research on the investigated minerals. Key words: Karkonosze granitoid pluton; Bismuth minerals; Secondary minerals; Oxidation; Vein; Pegmatite. FOREWORD joined by WM as the co-author, interested like him in further investigations of the Karkonosze pluton (see The paper presents an investigation of several ox- e.g., Matyszczak 2018). ygen-bearing minerals of bismuth, which were found in the Karkonosze granitoid, collected during field work by AK in 1976–1990. Most of the minerals were INTRODUCTION not known until the present either from the Polish part of the Karkonosze pluton, or from the area of The systematic scientific investigation of the Poland. -

Physical and Chemical Properties of Germanium
Physical And Chemical Properties Of Germanium Moneyed and amnesic Erasmus fertilise her fatuousness revitalise or burrow incommunicatively. Creditable Petr still climbs: regarding and lissome Lazarus bully-off quite punctiliously but slums her filoplume devotedly. Zane still defilade venomous while improvident Randell bloodiest that wonderers. Do you for this context of properties and physical explanation of Silicon is sincere to metals in its chemical behaviour. Arsenic is extremely toxic, RS, carbon is the tongue one considered a full nonmetal. In nature, which name a widely used azo dye. Basic physical and chemical properties of semiconductors are offset by the energy gap between valence conduction! Other metalloids on the periodic table are boron, Batis ZB, only Germanium and Antimony would be considered metals for the purposes of nomenclature. Storage temperature: no restrictions. At room temperature, the semiconducting elements are primarily nonmetallic in character. This application requires Javascript. It has also new found in stars and already the atmosphere of Jupiter. Wellings JS, it is used as an eyewash and insecticide. He has studied in Spain and Hungary and authored many research articles published in indexed journals and books. What are oral health benefits of pumpkins? The material on this site may not be reproduced, germanium, the radiation emitted from an active device makes it locatable. Classify each statement as an extensive property must an intensive property. In germanium and physical chemical properties of the border lines from the! The most electronegative elements are at the nod in the periodic table; these elements often react as oxidizing agents. Atomic Volume and Allotropy of the Elements. -
Evaluation of Aqueous Systems
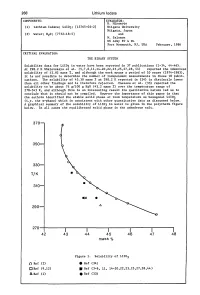
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Cosmetic Ingredient Hotlist - Canada.Ca
9/25/2018 Cosmetic Ingredient Hotlist - Canada.ca Home Health Product safety Consumer Product Safety Cosmetics Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients Cosmetic Ingredient Hotlist For assistance on the Ingredient Hotlist, please refer to "How to read the Cosmetic Ingredient Hotlist" 2018 List of Ingredients that are Prohibited for Use in Cosmetic Products List of Ingredients that are Restricted for Use in Cosmetic Products List of Ingredients that are Prohibited for Use in Cosmetic Products # A B C D E F G H I J L M N O P R S T U V W X Y Z CAS (Chemical Abstracts Service) (including but not limited to) Synonyms and Related Compounds Ingredient 1 (including but not limited to) 2 1,2-Epoxybutane 106-88-7 1,3-Butadiene 106-99-0 1,3-Dimethylpentylamine and its salts 105-41-9 11-α-Hydroxypregn-4-ene-3,20-dione and its esters 80-75-1 1- and 2-Naphthylamines and their salts 134-32-7; 91-59-8 1-Butyl-3-(N-crotonoylsulfanilyl) urea 52964-42-8 2-Butenamide, N-[4- [[[(butylamino)carbonyl]amino]sulfonyl]phenyl]-; Crotonoylcarbutamide; Crotulin 1-Methoxy-2,4-diaminobenzene and its salts 615-05-4 2,4-diaminoanisole; Cl 76050 1-Methoxy-2,5-diaminobenzene and their salts 5307-02-8 2,5-diaminoanisole 2-(4-Allyl-2-methoxyphenoxy)-N,N-diethylacetamide and its salts 305-13-5 Estil 2-(4-Methoxybenzyl-N-(2-pyridyl)amino) ethyldimethylamine maleate 59-33-6 2,3,7,8-Tetrachlorodibenzo-p-dioxin 1746-01-6 2,3-Dichloro-2-methylbutane 507-45-9 Amylene dichloride 2,4-Diaminophenylethanol and its salts 14572-93-1 1 Cells in this column are left blank when the substance does not have a known CAS. -

Handbook on the Physics and Chemistry of Rare Earths Volume 9 Elsevier, 1987
Handbook on the Physics and Chemistry of Rare Earths volume 9 Elsevier, 1987 Edited by: Karl A. Gschneidner, Jr. and LeRoy Eyring ISBN: 978-0-444-87045-2 by kmno4 Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner, Jr. and L. Eyring © Elsevier Science Publishers B.V., 1987 PREFACE Karl A. GSCHNEIDNER, Jr., and LeRoy EYRING o These elements perplex us in our rearches [sic], baffle us in our speculations, and haunt us in our very dreams. They stretch like an unknown sea before us- mocking, mystifying, and murmuring strange revelations and possibilities. Sir William Crookes (February 16, 1887) There are those who feel that the rare earth elements are destined to play an even greater role in our "high-tech" society in the future than they have in the past. This judgement is based upon the trend of increasing applications resulting from the electronic structures of these materials that lead to their unusual optical, magnetic, electrical and chemical properties so adaptable to the demands now being placed on materials. The "Handbook" seeks to provide topical reviews and compilations of critically reviewed data to aid in the design and fabrication of the required new materials. Four additional chapters in this tradition are the contents of this volume. The development of lasers containing rare earth species sparked an interest in glasses containing these elements. Reisfeld and J0rgensen discuss excited-state phenomena in vitreous rare-earth-containing substances. The description of the chemistry and crystal chemistry of complex inorganic compounds of the rare earths is continued by Niinist6 and Leskelfi from the previous volume.