Delayed Ettringite Formation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sulphoaluminates of Calcium
RPS4 THE SULPHOALUMINATES OF CALCIUM By William Lerch, F. W. Ashton, and R. H. Bogue ABSTRACT Two calcium sulphoaluminates have been prepared and their compositions and optical characteristics determined. One of these, having the composition 3CaO.Al2 3 .3CaS04 .31H 2 0, is relatively stable in aqueous solutions but is decom- posed by solutions of magnesium salts and of carbonates. The other, having the composition 3CaO.Al2 3 .CaS04.12H 2 0, is relatively unstable except in high concentration of hydroxyl ion. It is readily converted to the high sulphate form in aqueous solutions but is decomposed by magnesium salts and carbonates. In sulphate solutions the high sulphate form may be produced, but in the presence of a slight excess of magnesium salts or carbonates neither of the sulphoaluminates can continue to exist. In these cases the less soluble magnesium hydroxide and calcium carbonate, respectively, are formed. CONTENTS Page I. Introduction 715 II. Review of the literature 716 III. Experimental procedure 718 1. The high sulphate calcium sulphoaluminate 718 2. The low sulphate calcium sulphoaluminate 722 3. Calcium sulphosilicoaluminate 724 IV. Conditions of stability in salt solutions 725 V. Relative stability with calcium chloraluminate 729 VI. Summary 729 L INTRODUCTION Among the products formed by the action of sulphate waters on Portland cement there has been observed, under some conditions, a calcium sulphoaluminate. This has been ascribed by some inves- tigators to be responsible for certain expansion effects observed at times in concrete which is exposed to sea water and to alkali soil waters. The exact nature of this compound and its conditions of formation and stability have not been adequately treated, however. -

Manuscript Details
Manuscript Details Manuscript number MATERIALSCHAR_2019_439_R1 Title THE EFFECTS OF MgO, Na2O AND SO3 ON INDUSTRIAL CLINKERING PROCESS: PHASE COMPOSITION, POLYMORPHISM, MICROSTRUCTURE AND HYDRATION, USING A MULTIDISCIPLINARY APPROACH. Article type Research Paper Abstract The present investigation deals with how minor elements (their oxides: MgO, Na2O and SO3) in industrial kiln feeds affect (i) chemical reactions upon clinkering, (ii) resulting phase composition and microstructure of clinker, (iii) hydration process during cement production. Our results show that all these points are remarkably sensitive to the combination and interference effects between the minor chemical species mentioned above. Upon clinkering, all the industrial raw meals here used exhibit the same formation temperature and amount of liquid phase. Minor elements are preferentially hosted by secondary phases, such as periclase. Conversely, the growth rate of the main clinker phases (alite and belite) is significantly affected by the nature and combination of minor oxides. MgO and Na2O give a very fast C3S formation rate at T > 1450 K, whereas Na2O and SO3 boost C2S. After heating, if SO3 occurs in combination with MgO and/or Na2O, it does not inihibit the C3S crystallisation as expected. Rather, it promotes the stabilisation of M1-C3S, thus indirectly influencing the aluminate content, too. MgO increseases the C3S amount and promotes the stabilisation of M3-C3S, when it is in combination with Na2O. Na2O seems to be mainly hosted by calcium aluminate structure, but it does not induce the stabilisation of the orhtorhombic polymorph, as supposed to occur. Such features play a key role in predicting the physical-mechanical performance of a final cement (i.e. -
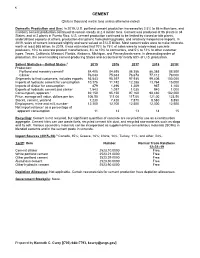
Cement Data Sheet
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

96 Quality Control of Clinker Products by SEM and XRF Analysis Ziad Abu
ACXRI '96 Quality Control of Clinker Products By SEM and XRF Analysis Ziad Abu Kaddourah and Khairun Azizi MY9700786 School of Materials and Mineral Resources Eng., Universiti Sains Malaysia 31750 Tronoh, Perak, Malaysia. ABSTRACT The microstructure and chemical properties of industrial Portland cement clinkers have been examined by SEM and XRF methods to establish the nature of the clinkers and how variations in the clinker characteristics can be used to control the clinker quality. The clinker nodules were found to show differences in the chemical composition and microstructure between the inner and outer parts of the clinker nodules. Microstructure studies of industrial Portland cement clinker have shown that the outer part of the nodules are enriched in silicate more than the inner part. There is better crystallization and larger alite crystal 9ize in the outer part than in the inner part. The alite crystal size varied between 16.2-46.12um. The clinker chemical composition was found to affect the residual >45um, where a higher belite content causes an increase in the residual >45um in the cement product and will cause a decrease in the concrete strength of the cement product. The aluminate and ferrite crystals and the microcracks within the alite crystal are clear in some clinker only. The quality of the raw material preparation, burning and cooling stages can be controlled using the microstructure of the clinker product. INTRODUCTION Examination of manufactured industrial clinkers using the Scanning Electron Microscope (SEM) is usually conducted to study problems that can't be defined by the normal quality control procedures. Such a study can be used to give better information and knowledge about clinkers characteristics and how variations in the clinker characteristics are affected by variations in the various stages during the manufacturing process. -

Industrial Scale Kiln Problems and Their Solution with Controlling Different Operating Parameters
Open Access Austin Chemical Engineering Research Article Industrial Scale Kiln Problems and Their Solution with Controlling Different Operating Parameters Fazeel Ahmad* Department of Chemical Engineering, University of Wah, Abstract Wah Engineering College, Pakistan The chemical composition homogeneity of kiln feed has an important *Corresponding author: Fazeel Ahmad, Department relationship to fuel consumption, kiln operation, clinker formation and cement of Chemical Engineering, University of Wah, Wah strength. Kiln operation can be made stable and smooth. The basis for this Engineering College, Pakistan property is a well-burned clinker with consistent chemical composition and free lime. The main reason for the clinker free lime to change in situation with Received: April 19, 2020; Accepted: May 12, 2020; stable kiln operation is variation in the chemical composition of the kiln feed. Published: May 19, 2020 This variation in chemical composition is related to raw mix control and the homogenization process. To ensure a constant quality of the product and maintain a stable and continuous operation of the kiln, the attention must be paid to storage and homogenization of raw materials and kiln feed. Due to variations in the kiln feed chemical compositions that affect its burn ability and the fuel consumption. The objective of this research paper is to provide solutions of the problems occurring in well burned clinker through stable kiln operation and efficient cooler heat transfer. The quality of cement is assessed typically from its -

Thermodynamics of Portland Cement Clinkering
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aberdeen University Research Archive Thermodynamics of Portland Cement Clinkering Theodore Hanein1, Fredrik P. Glasser2, Marcus Bannerman1* 1. School of Engineering, University of Aberdeen, AB24 3UE, United Kingdom 2. Department of Chemistry, University of Aberdeen, AB24 3UE, United Kingdom Abstract The useful properties of cement arise from the assemblage of solid phases present within the cement clinker. The phase proportions produced from a given feedstock are often predicted using well-established stoichiometric relations, such as the Bogue equations. These approaches are based on a single estimation of the stable phases produced under standard processing conditions and so they are limited in their general application. This work presents a thermodynamic database and simple equilibrium model which is capable of predicting cement phase stability across the full range of kiln temperatures, including the effect of atmospheric conditions. This is termed a “reaction path” and benchmarks the kinetics of processes occurring in the kiln. Phase stability is calculated using stoichiometric phase data and Gibbs free energy minimization under the constraints of an elemental balance. Predictions of stable phases and standard phase diagrams are reproduced for the manufacture of ordinary Portland cement and validated against results in the literature. The stability of low-temperature phases, which may be important in kiln operation, is explored. Finally, an outlook on future applications of the database in optimizing cement plant operation and development of new cement formulations is provided. Originality This report details corrections to reference thermodynamic data which are relevant for cement thermodynamic calculations. -

Cement Heat of Hydration and Thermal Control Ahmadreza Sedaghat
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 3-22-2016 Cement Heat of Hydration and Thermal Control Ahmadreza Sedaghat Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the Civil Engineering Commons, and the Materials Science and Engineering Commons Scholar Commons Citation Sedaghat, Ahmadreza, "Cement Heat of Hydration and Thermal Control" (2016). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/6142 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Cement Heat of Hydration and Thermal Control by Ahmadreza Sedaghat A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Civil and Environmental Engineering College of Engineering University of South Florida Major Professor: A. Zayed, Ph.D. Manoj K. Ram, Ph.D. Mahmoood H. Nachabe, Ph.D. Ajit Mujumdar, Ph.D. Abdul Malik, Ph.D. Date of Approval: January 29, 2016 Keywords: Portland Cement, Isothermal Calorimetry, Graphene Nanoplatelet, Statistical Analysis, Thermal Cracking Copyright © 2016, Ahmadreza Sedaghat DEDICATION I would like to dedicate my dissertation to my parents (Aliasghar Sedaghat, Golnar J. Javidan), my beloved wife Rana, my brother Arsalan and my grandmother Ms. Koukab Sanakhan. A special feeling of gratitude to my parents whose words of encouragement and push for tenacity ring in my ears. My brother Arsalan who supported me emotionally and was a strong pier for my parents to lean on while I was far away from home pursuing my education. -

Portland Cement Clinker
Conforms to HazCom 2012/United States Safety Data Sheet Portland Cement Clinker Section 1. Identification GHS product identifier: Portland Cement Clinker Chemical name: Calcium compounds, calcium silicate compounds, and other calcium compounds containing iron and aluminum make up the majority of this product. Other means of identification: Clinker, Cement Clinker Relevant identified uses of the substance or mixture and uses advised against: Raw material for cement manufacturing. Supplier’s details: 300 E. John Carpenter Freeway, Suite 1645 Irving, TX 75062 (972) 653-5500 Emergency telephone number (24 hours): CHEMTREC: (800) 424-9300 Section 2. Hazards Identification Overexposure to portland cement clinker can cause serious, potentially irreversible skin or eye damage in the form of chemical (caustic) burns, including third degree burns. The same serious injury can occur if wet or moist skin has prolonged contact exposure to dry portland cement clinker. OSHA/HCS status: This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the SKIN CORROSION/IRRITATION – Category 1 substance or mixture: SERIOUS EYE DAMAGE/EYE IRRITATION – Category 1 SKIN SENSITIZATION – Category 1 CARCINOGENICITY/INHALATION – Category 1A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) [Respiratory tract irritation] – Category 3 GHS label elements Hazard pictograms: Signal word: Danger Hazard statements: Causes severe skin burns and eye damage. May cause an allergic skin reaction. May cause respiratory irritation. May cause cancer. Precautionary statements: Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing dust. Use outdoors in a well ventilated area. Wash any exposed body parts thouroughly after handling. -

Separate Calcination in Cement Clinker Production
Separate Calcination in Cement Clinker Production A laboratory scale study on how an electrified separate calcination step affects the phase composition of cement clinker Amanda Vikström Master thesis, 30 hp M.Sc. in Energy Engineering, 300 hp Department of Applied Physics and Electronics, Spring term 2021 Separate Calcination in Cement Clinker Production A laboratory scale study on how an electrified separate calcination step affects the phase composition of cement clinker Amanda Vikström [email protected] Master Thesis in Energy Engineering Department of Applied Physics and Electronics Umeå University Examiner: Robert Eklund Supervisors: Matias Eriksson, José Aguirre Castillo and Bodil Wilhelmsson Performed in collaboration with Cementa AB and the Centre for Sustainable Cement and Quicklime Production at Umeå University 31 may 2021 Abstract Cement production is responsible for around 7% of the global anthropogenic carbon dioxide emissions. More than half of these emissions are due to the unavoidable release of carbon dioxide upon thermal decomposition of the main raw material limestone. Many different options for carbon capture are currently being investigated to lower emissions, and one potential route to facilitate carbon capture could be the implementation of an electrified separate calcination step. However, potential effects on the phase composition of cement clinker need to be investigated, which is the aim of the present study. Phases of special interest are alite, belite, aluminate, ferrite, calcite, and lime. The phase composition during clinker formation was examined through HT-XRD lab-scale experiments, allowing the phase transformations to be observed in situ. Two different methods of separate calcination were investigated, one method in which the raw meal was calcined separately, and one method where the limestone was calcined separately. -

Equivalent Cement Clinker Obtained by Indirect Mechanosynthesis Process
materials Article Equivalent Cement Clinker Obtained by Indirect Mechanosynthesis Process Rabah Hamzaoui * and Othmane Bouchenafa * Institut de Recherche en Constructibilité IRC, ESTP, Université Paris-Est, 28 Avenue du Président Wilson, 94234 Cachan, France * Correspondence: [email protected] (R.H.); [email protected] (O.B.); Tel.: +33149080334 (R.H.); +33149082327 (O.B.) Received: 23 September 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: The aim of this work is to study the heat treatment effect, milling time effect and indirect mechanosynthesis effect on the structure of the mixture limestone/clay (kaolinite). Indirect mechanosynthesis is a process that combines between mechanical activation and heat treatment at 900 ◦C. XRD, TGA, FTIR and particle size distribution analysis and SEM micrograph are used in order to follow thermal properties and structural modification changes that occur. It is shown that the indirect mechanosynthesis process allows the formation of the equivalent clinker in powder with the main constituents of the clinker (Alite C3S, belite C2S, tricalcium aluminate C3A and tetracalcium aluminoferrite C4AF) at 900 ◦C, whereas, these constituents in the conventional clinker are obtained at 1450 ◦C. Keywords: cement clinker; clinkerization; indirect mechanosynthesis; nanostructured materials; crystalline structures 1. Introduction Portland cement is manufactured from raw material obtained by mixing and grinding limestone and minerals rich in silica and alumina (clay or kaolin) and other additives. This mixture is then calcined at 1450 ◦C to obtain the clinker. The clinker is finely crushed and blended with a source of sulfate (gypsum or anhydrite) and other minerals to form the cement [1]. However, the production of cement is responsible for high energy consumption. -

Calcium Sulfate Precipitation Throughout Its Phase Diagram
Chapter 12 Calcium Sulfate Precipitation Throughout Its Phase Diagram Alexander E.S. Van Driessche, Tomasz M. Stawski, Liane G. Benning, and Matthias Kellermeier 12.1 Introduction Over the past decade, significant progress has been made in our understanding of crystallization phenomena. In particular, a number of precursor and intermediate species, both solutes and solids, and either stable or metastable (or even unstable), have been identified. Their existence extends the simplified picture of classical nucleation and growth theories toward much more complex pathways. These newly found species include various solute clusters, liquid-like phases, amorphous particles, and metastable (often nanosized) crystalline polymorphs, all of which may exist for different periods and may convert into one another depending on the chosen conditions (see, for instance, De Yoreo et al. 2017, Chap. 1; Wolf and Gower 2017, Chap. 3; Rodriguez-Blanco et al. 2017, Chap. 5; Birkedal 2017, Chap. 10; Reichel and Faivre 2017, Chap. 14, and references therein). Quite naturally, most A.E.S. Van Driessche Driessche () University Grenoble Alpes, CNRS, ISTerre, F-38000 Grenoble, France e-mail: [email protected] T.M. Stawski German Research Centre for Geosciences, GFZ, D-14473 Potsdam, Germany School of Earth and Environment, University of Leeds, LS2 9JT Leeds, UK L.G. Benning German Research Center for Geosciences, GFZ, Interface Geochemistry Section, 14473 Potsdam, Germany Department of Earth Sciences, Free University of Berlin, 12249 Berlin, Germany School of Earth and Environment, University of Leeds, Leeds LS2 9JT, UK M. Kellermeier () Material Physics, BASF SE, Carl-Bosch-Str. 38, D-67056 Ludwigshafen, Germany e-mail: [email protected] © Springer International Publishing Switzerland 2017 227 A.E.S.