Thermodynamics of Portland Cement Clinkering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manuscript Details
Manuscript Details Manuscript number MATERIALSCHAR_2019_439_R1 Title THE EFFECTS OF MgO, Na2O AND SO3 ON INDUSTRIAL CLINKERING PROCESS: PHASE COMPOSITION, POLYMORPHISM, MICROSTRUCTURE AND HYDRATION, USING A MULTIDISCIPLINARY APPROACH. Article type Research Paper Abstract The present investigation deals with how minor elements (their oxides: MgO, Na2O and SO3) in industrial kiln feeds affect (i) chemical reactions upon clinkering, (ii) resulting phase composition and microstructure of clinker, (iii) hydration process during cement production. Our results show that all these points are remarkably sensitive to the combination and interference effects between the minor chemical species mentioned above. Upon clinkering, all the industrial raw meals here used exhibit the same formation temperature and amount of liquid phase. Minor elements are preferentially hosted by secondary phases, such as periclase. Conversely, the growth rate of the main clinker phases (alite and belite) is significantly affected by the nature and combination of minor oxides. MgO and Na2O give a very fast C3S formation rate at T > 1450 K, whereas Na2O and SO3 boost C2S. After heating, if SO3 occurs in combination with MgO and/or Na2O, it does not inihibit the C3S crystallisation as expected. Rather, it promotes the stabilisation of M1-C3S, thus indirectly influencing the aluminate content, too. MgO increseases the C3S amount and promotes the stabilisation of M3-C3S, when it is in combination with Na2O. Na2O seems to be mainly hosted by calcium aluminate structure, but it does not induce the stabilisation of the orhtorhombic polymorph, as supposed to occur. Such features play a key role in predicting the physical-mechanical performance of a final cement (i.e. -
Cement Data Sheet
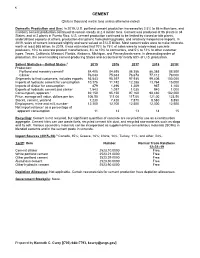
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

96 Quality Control of Clinker Products by SEM and XRF Analysis Ziad Abu
ACXRI '96 Quality Control of Clinker Products By SEM and XRF Analysis Ziad Abu Kaddourah and Khairun Azizi MY9700786 School of Materials and Mineral Resources Eng., Universiti Sains Malaysia 31750 Tronoh, Perak, Malaysia. ABSTRACT The microstructure and chemical properties of industrial Portland cement clinkers have been examined by SEM and XRF methods to establish the nature of the clinkers and how variations in the clinker characteristics can be used to control the clinker quality. The clinker nodules were found to show differences in the chemical composition and microstructure between the inner and outer parts of the clinker nodules. Microstructure studies of industrial Portland cement clinker have shown that the outer part of the nodules are enriched in silicate more than the inner part. There is better crystallization and larger alite crystal 9ize in the outer part than in the inner part. The alite crystal size varied between 16.2-46.12um. The clinker chemical composition was found to affect the residual >45um, where a higher belite content causes an increase in the residual >45um in the cement product and will cause a decrease in the concrete strength of the cement product. The aluminate and ferrite crystals and the microcracks within the alite crystal are clear in some clinker only. The quality of the raw material preparation, burning and cooling stages can be controlled using the microstructure of the clinker product. INTRODUCTION Examination of manufactured industrial clinkers using the Scanning Electron Microscope (SEM) is usually conducted to study problems that can't be defined by the normal quality control procedures. Such a study can be used to give better information and knowledge about clinkers characteristics and how variations in the clinker characteristics are affected by variations in the various stages during the manufacturing process. -

Portland Cement Clinker
Conforms to HazCom 2012/United States Safety Data Sheet Portland Cement Clinker Section 1. Identification GHS product identifier: Portland Cement Clinker Chemical name: Calcium compounds, calcium silicate compounds, and other calcium compounds containing iron and aluminum make up the majority of this product. Other means of identification: Clinker, Cement Clinker Relevant identified uses of the substance or mixture and uses advised against: Raw material for cement manufacturing. Supplier’s details: 300 E. John Carpenter Freeway, Suite 1645 Irving, TX 75062 (972) 653-5500 Emergency telephone number (24 hours): CHEMTREC: (800) 424-9300 Section 2. Hazards Identification Overexposure to portland cement clinker can cause serious, potentially irreversible skin or eye damage in the form of chemical (caustic) burns, including third degree burns. The same serious injury can occur if wet or moist skin has prolonged contact exposure to dry portland cement clinker. OSHA/HCS status: This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the SKIN CORROSION/IRRITATION – Category 1 substance or mixture: SERIOUS EYE DAMAGE/EYE IRRITATION – Category 1 SKIN SENSITIZATION – Category 1 CARCINOGENICITY/INHALATION – Category 1A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) [Respiratory tract irritation] – Category 3 GHS label elements Hazard pictograms: Signal word: Danger Hazard statements: Causes severe skin burns and eye damage. May cause an allergic skin reaction. May cause respiratory irritation. May cause cancer. Precautionary statements: Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing dust. Use outdoors in a well ventilated area. Wash any exposed body parts thouroughly after handling. -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Cementing Qualities of the Calcium Aluminates
DEPARTMENT OF COMMERCE Technologic Papers of THE Bureau of Standards S. W. STRATTON, Director No. 19 7 CEMENTING QUALITIES OF THE CALCIUM ALUMINATES BY P. H. BATES, Chemist Bureau of Standards SEPTEMBER 27, 1921 PRICE, 10 CENTS Sold only by the Superintendent of Documents, Government Printing Office Washington, D. C. WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CEMENTING QUALITIES OF THE CALCIUM ALBUMI- NATES Bv P. H. Bates ABSTRACT four calcium aluininates (3CaO.Al 5Ca0.3Al , CaO.Al The 2 3 , 2 3 2 3 , 3Ca0.5Al 2 3 ) have been made in a pure condition and their cementing qualities determined. The first two reacted so energetically with water that too rapid set resulted to make them usable commercially. The last two set more slowly and developed very high strengths at early periods. These two alurninates high in alumina were later made in a pure and impure condition in larger quantities in a rotary kiln and concrete was made from the resulting ground clinker. A 1:1.5:5.5 gravel concrete developed in 24 hours as high strength as a similarly proportioned Portland cement concrete would have developed in 28 days. Recent investigations * have shown that lime can combine with alumina only in the following molecular proportions: 3CaO. A1 5Ca0.3Al , CaO.Al 3Ca0.5Al . These investiga- 2 3 , 2 3 2 3 , 2 3 tions also show that the tricalcium aluminate is the only aluminate present in Portland cement of normal composition and normal properties. Later these results were confirmed by work carried on at this Bureau and published as Technologic Paper No. -

Morphological Analysis of White Cement Clinker Minerals: Discussion on the Crystallization-Related Defects
Hindawi Publishing Corporation International Journal of Analytical Chemistry Volume 2016, Article ID 1259094, 10 pages http://dx.doi.org/10.1155/2016/1259094 Research Article Morphological Analysis of White Cement Clinker Minerals: Discussion on the Crystallization-Related Defects Mohamed Benmohamed,1,2 Rabah Alouani,2 Amel Jmayai,1 Abdesslem Ben Haj Amara,1 and Hafsia Ben Rhaiem1 1 UR05/13-01, Physique des Materiaux´ Lamellaires et Nanomateriaux´ Hybrides (PMLNMH), Faculte´ des Sciences de Bizerte, 7021 Zarzouna, Tunisia 2Departement´ de Geologie,´ Faculte´ des Sciences de Bizerte, Zarzouna, 7021 Bizerte, Tunisia Correspondence should be addressed to Mohamed Benmohamed; [email protected] Received 23 January 2016; Accepted 28 April 2016 Academic Editor: Valentina Venuti Copyright © 2016 Mohamed Benmohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The paper deals with a formation of artificial rock (clinker). Temperature plays the capital role in the manufacturing process. So, itis useful to analyze a poor clinker to identify the different phases and defects associated with their crystallization. X-ray fluorescence spectroscopy was used to determine the clinker’s chemical composition. The amounts of the mineralogical phases are measured by quantitative XRD analysis (Rietveld). Scanning electron microscopy (SEM) was used to characterize the main phases of white Portland cement clinker and the defects associated with the formation of clinker mineral elements. The results of a study which focused on the identification of white clinker minerals and defects detected in these noncomplying clinkers such as fluctuation of the amount of the main phases (alite (C3S) and belite (C2S)), excess of the free lime, occurrence of C3S polymorphs, and occurrence of moderately-crystallized structures are presented in this paper. -

Portland Cements. in This Lesson, We Will Discuss the Physical and Chemical Properties of Portland Cement
Welcome to the Highway Materials Engineering Course Module G, Lesson 2: Portland Cements. In this lesson, we will discuss the physical and chemical properties of Portland cement. A printer‐friendly version of the lesson materials can be downloaded by selecting the paperclip icon. Only the screens for the this lesson are available. If you need technical assistance during the training, please select the Help link in the upper right‐hand corner of the screen. By the end of this lesson, you will be able to: • Explain the effect of different types of cement on plastic and hardened Portland cement concrete (PCC) properties; • Describe the production process for Portland cement; • Relate the chemical and physical properties of Portland cement to the concrete; and • Evaluate chemical and physical properties of Portland cement. During this lesson, knowledge checks are provided to test your understanding of the material presented. This lesson will take approximately 60 minutes to complete. Let’s begin with identifying the primary cement types and how they differ. Select each type for more information. • Type I; • Type II; • Type III; • Type IV; • Type V; • Types IA, IIA, and IIIA; • Type II (MH); • Type II (MH)A; and • Type K. Note that Type I/II Portland cement is not an official designation but is widely available and used extensively in transportation systems. Type I/II cement meets the specifications for both Type I and Type II cements but has a lower alkali content for use where reactive aggregates are found. Use of Type I/II cement alone is not sufficient to prevent alkali‐silica reactivity (ASR). -

White Cement - Properties, Manufacture, Prospects
Review paper WHITE CEMENT - PROPERTIES, MANUFACTURE, PROSPECTS KARTEŘINA MORESOVÁ, FRANTIŠEK ŠKVÁRA Department of Glass and Ceramics, Institute of Chemical Technology, Technická 5, 166 28 Prague 6, Czech Republic E-mail: [email protected] Submitted January 6, 2000; accepted March 12, 2001. Keywords: White cement, Review, Properties INTRODUCTION stage and must also exhibit whiteness corresponding to cement of the given class. The raw materials, the Special cements differ from conventional Portland intermediate product and the final product must also be cements in their chemical and phase composition as protected from contamination at all stages of the well as in their properties. Their properties can be technology. The initial composition of the raw materials achieved by using a modified raw material mix, a or their properties are dealt with e.g. by [9 - 16]. grinding admixture or by adjusting the grinding fineness White cement may be manufactured with of the cement. Special cements include a number of standardized surface-active plasticizers or hydrophobic cements whose production volumes are considerably additives (in amounts of up to 0.5 wt.%) not impairing lower compared to those of conventional cements (max. the whiteness of the product. According to the 5 to 10 %). However, they constitute a significant part whiteness degree, white cement is divided into three of the assortment supplementing the conventional groups of the reflection coefficient: I. min. 80 %, II. cement. These materials include cements with high min. 75 % and III. min. 68 %. The whiteness degree is early strengths, sulphate-resistant Portland cements, determined according to the reflection coefficient in road cements, white cement and coloured cements, percent of the absolute scale. -

Sources of Mercury, Behavior in Cement Process and Abatement Options
Sources of mercury, behavior in cement process and abatement options Volker Hoenig European Cement Research Academy Cement Industry Sector Partnership on Mercury Partnership Launch Meeting Geneva, 18/19 June 2013 Agenda 1 Cement production process 2 Behaviour of mercury in Cement Production Process 3 Mercury abatement techniques 4 Monitoring of mercury emissions 5 Mercury emission inventories 6 BestEmissions Available inventories Technique and als Extrapunkt Best Environmental Practice Bedenken 1 Cement production process General principle: • Raw materials: limestone, clay, lime marl • Thermal treatment to produce cement clinker (1450°C) Commonly in rotary cement kilns Regular fuels: black coal, lignite, petroleum coke, natural gas, heavy fuel Alternative fuels: plastics, mixed industrial wastes (RDF), tyres, … 1 Cement production process Exhaust gas utilization • Kiln exhaust gases are mainly used conditioning for drying of raw materials ESP/FF tower • Two modes of operation: raw mill on / raw mill off • Raw mill gas stream determined by required heat ->raw material moisture • Residual kiln exhaust stream preheater bypasses raw mill and is conditioned in evaporating cooler • Example: dry raw materials kiln Less exhaust gas needed for drying raw mill More exhaust gas bypasses raw mill • Ratio raw mill on/off operation mainly determined by ratio raw mill/kiln capacity 1 Cement production process Dust utilization • Prepicipated dust consists mainly of conditioning raw material ESP/FF tower • Raw mill on: „dust“ is ground raw material -

The Durability of White Portland Cement to Chemical Attack
Downloaded from orbit.dtu.dk on: Dec 18, 2017 The durability of white Portland cement to chemical attack Nielsen, Erik Pram; Geiker, Mette Rica Publication date: 2004 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Nielsen, E. P., & Geiker, M. R. (2004). The durability of white Portland cement to chemical attack. (BYG- Rapport; No. R-084). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Erik Pram Nielsen The durability of white Portland cement to chemical attack DANMARKS TEKNISKE UNIVERSITET Report BYG·DTU R-084 2004 ISSN 1601-2917 ISBN 87-7877-147-1 SUMMARY In terms of volume, the severest conditions that reinforced concrete structures are subjected to usually involve some form of attack by chlorides. These include marine environments, roads and bridges exposed to de-icing salt, etc. The time it takes for the chlorides to reach the reinforcement is the main parameter used to predict the service life of the concrete structures. -

Istra 40 Aggregates for High Performance Concrete Application of CALUCEM – Calcium Aluminate Cements
Building Chemistry | Construction Industry | Pipes & Aggregates | Refractory Industry | Reprocessing Istra 40 Aggregates for High Performance Concrete Application of CALUCEM – Calcium Aluminate Cements INTRODUCTION Where can ISTRA 40 AGG be used for mortars and concrete? Areas of application are: Mortars and concretes with ISTRA 40 AGG are ideally suited for aggressive environments where durability is of utmost importance and Industrial floorings: When used in combination with ISTRA Ordinary Portland Cement concrete fails. ISTRA 40 AGG, when mixed Calcium Aluminate Cement, ISTRA 40 AGG provides a hard and dense with ISTRA Calcium Aluminate Cement, offers superior performance overall matrix, allowing high mechanical loading. The composite against abrasion, chemical attack, high temperatures, mechanical hardening effect ensures that the service life of the floor will last longer impact and thermal shock. under constant exposure to heavy duty attacks i.e. high point load, acids, animal fats, oils and industrial wastes. Pressure sewers and pipes: Combination of ISTRA Calcium Aluminate Cement with ISTRA 40 AGG increases the abrasion resistance of mortar lining particularly in areas of high turbulence along the sewage networks. Hydraulic structures: ISTRA 40 AGG is a sound material to be used in areas of high abrasion i.e. flushing gates and spill ways. It provides excellent protection from water erosion in high wear zones. High temperature tolerance places: ISTRA 40 AGG has found usage in furnace floors, slag pits and storage facilities in metallurgical industries where temperature requirements could reach up to 1200°C. ISTRA 40 AGG has proven to be stable in the conditions of extreme thermal cycling from below zero to 1000°C.