Improving Concrete Sustainability Through Alite and Belite Reactivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manuscript Details
Manuscript Details Manuscript number MATERIALSCHAR_2019_439_R1 Title THE EFFECTS OF MgO, Na2O AND SO3 ON INDUSTRIAL CLINKERING PROCESS: PHASE COMPOSITION, POLYMORPHISM, MICROSTRUCTURE AND HYDRATION, USING A MULTIDISCIPLINARY APPROACH. Article type Research Paper Abstract The present investigation deals with how minor elements (their oxides: MgO, Na2O and SO3) in industrial kiln feeds affect (i) chemical reactions upon clinkering, (ii) resulting phase composition and microstructure of clinker, (iii) hydration process during cement production. Our results show that all these points are remarkably sensitive to the combination and interference effects between the minor chemical species mentioned above. Upon clinkering, all the industrial raw meals here used exhibit the same formation temperature and amount of liquid phase. Minor elements are preferentially hosted by secondary phases, such as periclase. Conversely, the growth rate of the main clinker phases (alite and belite) is significantly affected by the nature and combination of minor oxides. MgO and Na2O give a very fast C3S formation rate at T > 1450 K, whereas Na2O and SO3 boost C2S. After heating, if SO3 occurs in combination with MgO and/or Na2O, it does not inihibit the C3S crystallisation as expected. Rather, it promotes the stabilisation of M1-C3S, thus indirectly influencing the aluminate content, too. MgO increseases the C3S amount and promotes the stabilisation of M3-C3S, when it is in combination with Na2O. Na2O seems to be mainly hosted by calcium aluminate structure, but it does not induce the stabilisation of the orhtorhombic polymorph, as supposed to occur. Such features play a key role in predicting the physical-mechanical performance of a final cement (i.e. -
Cement Data Sheet
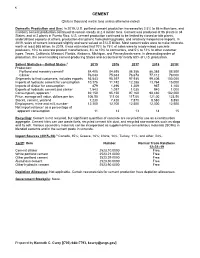
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

96 Quality Control of Clinker Products by SEM and XRF Analysis Ziad Abu
ACXRI '96 Quality Control of Clinker Products By SEM and XRF Analysis Ziad Abu Kaddourah and Khairun Azizi MY9700786 School of Materials and Mineral Resources Eng., Universiti Sains Malaysia 31750 Tronoh, Perak, Malaysia. ABSTRACT The microstructure and chemical properties of industrial Portland cement clinkers have been examined by SEM and XRF methods to establish the nature of the clinkers and how variations in the clinker characteristics can be used to control the clinker quality. The clinker nodules were found to show differences in the chemical composition and microstructure between the inner and outer parts of the clinker nodules. Microstructure studies of industrial Portland cement clinker have shown that the outer part of the nodules are enriched in silicate more than the inner part. There is better crystallization and larger alite crystal 9ize in the outer part than in the inner part. The alite crystal size varied between 16.2-46.12um. The clinker chemical composition was found to affect the residual >45um, where a higher belite content causes an increase in the residual >45um in the cement product and will cause a decrease in the concrete strength of the cement product. The aluminate and ferrite crystals and the microcracks within the alite crystal are clear in some clinker only. The quality of the raw material preparation, burning and cooling stages can be controlled using the microstructure of the clinker product. INTRODUCTION Examination of manufactured industrial clinkers using the Scanning Electron Microscope (SEM) is usually conducted to study problems that can't be defined by the normal quality control procedures. Such a study can be used to give better information and knowledge about clinkers characteristics and how variations in the clinker characteristics are affected by variations in the various stages during the manufacturing process. -

Industrial Scale Kiln Problems and Their Solution with Controlling Different Operating Parameters
Open Access Austin Chemical Engineering Research Article Industrial Scale Kiln Problems and Their Solution with Controlling Different Operating Parameters Fazeel Ahmad* Department of Chemical Engineering, University of Wah, Abstract Wah Engineering College, Pakistan The chemical composition homogeneity of kiln feed has an important *Corresponding author: Fazeel Ahmad, Department relationship to fuel consumption, kiln operation, clinker formation and cement of Chemical Engineering, University of Wah, Wah strength. Kiln operation can be made stable and smooth. The basis for this Engineering College, Pakistan property is a well-burned clinker with consistent chemical composition and free lime. The main reason for the clinker free lime to change in situation with Received: April 19, 2020; Accepted: May 12, 2020; stable kiln operation is variation in the chemical composition of the kiln feed. Published: May 19, 2020 This variation in chemical composition is related to raw mix control and the homogenization process. To ensure a constant quality of the product and maintain a stable and continuous operation of the kiln, the attention must be paid to storage and homogenization of raw materials and kiln feed. Due to variations in the kiln feed chemical compositions that affect its burn ability and the fuel consumption. The objective of this research paper is to provide solutions of the problems occurring in well burned clinker through stable kiln operation and efficient cooler heat transfer. The quality of cement is assessed typically from its -

Thermodynamics of Portland Cement Clinkering
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aberdeen University Research Archive Thermodynamics of Portland Cement Clinkering Theodore Hanein1, Fredrik P. Glasser2, Marcus Bannerman1* 1. School of Engineering, University of Aberdeen, AB24 3UE, United Kingdom 2. Department of Chemistry, University of Aberdeen, AB24 3UE, United Kingdom Abstract The useful properties of cement arise from the assemblage of solid phases present within the cement clinker. The phase proportions produced from a given feedstock are often predicted using well-established stoichiometric relations, such as the Bogue equations. These approaches are based on a single estimation of the stable phases produced under standard processing conditions and so they are limited in their general application. This work presents a thermodynamic database and simple equilibrium model which is capable of predicting cement phase stability across the full range of kiln temperatures, including the effect of atmospheric conditions. This is termed a “reaction path” and benchmarks the kinetics of processes occurring in the kiln. Phase stability is calculated using stoichiometric phase data and Gibbs free energy minimization under the constraints of an elemental balance. Predictions of stable phases and standard phase diagrams are reproduced for the manufacture of ordinary Portland cement and validated against results in the literature. The stability of low-temperature phases, which may be important in kiln operation, is explored. Finally, an outlook on future applications of the database in optimizing cement plant operation and development of new cement formulations is provided. Originality This report details corrections to reference thermodynamic data which are relevant for cement thermodynamic calculations. -

Portland Cement Clinker
Conforms to HazCom 2012/United States Safety Data Sheet Portland Cement Clinker Section 1. Identification GHS product identifier: Portland Cement Clinker Chemical name: Calcium compounds, calcium silicate compounds, and other calcium compounds containing iron and aluminum make up the majority of this product. Other means of identification: Clinker, Cement Clinker Relevant identified uses of the substance or mixture and uses advised against: Raw material for cement manufacturing. Supplier’s details: 300 E. John Carpenter Freeway, Suite 1645 Irving, TX 75062 (972) 653-5500 Emergency telephone number (24 hours): CHEMTREC: (800) 424-9300 Section 2. Hazards Identification Overexposure to portland cement clinker can cause serious, potentially irreversible skin or eye damage in the form of chemical (caustic) burns, including third degree burns. The same serious injury can occur if wet or moist skin has prolonged contact exposure to dry portland cement clinker. OSHA/HCS status: This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the SKIN CORROSION/IRRITATION – Category 1 substance or mixture: SERIOUS EYE DAMAGE/EYE IRRITATION – Category 1 SKIN SENSITIZATION – Category 1 CARCINOGENICITY/INHALATION – Category 1A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) [Respiratory tract irritation] – Category 3 GHS label elements Hazard pictograms: Signal word: Danger Hazard statements: Causes severe skin burns and eye damage. May cause an allergic skin reaction. May cause respiratory irritation. May cause cancer. Precautionary statements: Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing dust. Use outdoors in a well ventilated area. Wash any exposed body parts thouroughly after handling. -

Separate Calcination in Cement Clinker Production
Separate Calcination in Cement Clinker Production A laboratory scale study on how an electrified separate calcination step affects the phase composition of cement clinker Amanda Vikström Master thesis, 30 hp M.Sc. in Energy Engineering, 300 hp Department of Applied Physics and Electronics, Spring term 2021 Separate Calcination in Cement Clinker Production A laboratory scale study on how an electrified separate calcination step affects the phase composition of cement clinker Amanda Vikström [email protected] Master Thesis in Energy Engineering Department of Applied Physics and Electronics Umeå University Examiner: Robert Eklund Supervisors: Matias Eriksson, José Aguirre Castillo and Bodil Wilhelmsson Performed in collaboration with Cementa AB and the Centre for Sustainable Cement and Quicklime Production at Umeå University 31 may 2021 Abstract Cement production is responsible for around 7% of the global anthropogenic carbon dioxide emissions. More than half of these emissions are due to the unavoidable release of carbon dioxide upon thermal decomposition of the main raw material limestone. Many different options for carbon capture are currently being investigated to lower emissions, and one potential route to facilitate carbon capture could be the implementation of an electrified separate calcination step. However, potential effects on the phase composition of cement clinker need to be investigated, which is the aim of the present study. Phases of special interest are alite, belite, aluminate, ferrite, calcite, and lime. The phase composition during clinker formation was examined through HT-XRD lab-scale experiments, allowing the phase transformations to be observed in situ. Two different methods of separate calcination were investigated, one method in which the raw meal was calcined separately, and one method where the limestone was calcined separately. -

Equivalent Cement Clinker Obtained by Indirect Mechanosynthesis Process
materials Article Equivalent Cement Clinker Obtained by Indirect Mechanosynthesis Process Rabah Hamzaoui * and Othmane Bouchenafa * Institut de Recherche en Constructibilité IRC, ESTP, Université Paris-Est, 28 Avenue du Président Wilson, 94234 Cachan, France * Correspondence: [email protected] (R.H.); [email protected] (O.B.); Tel.: +33149080334 (R.H.); +33149082327 (O.B.) Received: 23 September 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: The aim of this work is to study the heat treatment effect, milling time effect and indirect mechanosynthesis effect on the structure of the mixture limestone/clay (kaolinite). Indirect mechanosynthesis is a process that combines between mechanical activation and heat treatment at 900 ◦C. XRD, TGA, FTIR and particle size distribution analysis and SEM micrograph are used in order to follow thermal properties and structural modification changes that occur. It is shown that the indirect mechanosynthesis process allows the formation of the equivalent clinker in powder with the main constituents of the clinker (Alite C3S, belite C2S, tricalcium aluminate C3A and tetracalcium aluminoferrite C4AF) at 900 ◦C, whereas, these constituents in the conventional clinker are obtained at 1450 ◦C. Keywords: cement clinker; clinkerization; indirect mechanosynthesis; nanostructured materials; crystalline structures 1. Introduction Portland cement is manufactured from raw material obtained by mixing and grinding limestone and minerals rich in silica and alumina (clay or kaolin) and other additives. This mixture is then calcined at 1450 ◦C to obtain the clinker. The clinker is finely crushed and blended with a source of sulfate (gypsum or anhydrite) and other minerals to form the cement [1]. However, the production of cement is responsible for high energy consumption. -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Alite Calcium Sulfoaluminate Cement: Chemistry and Thermodynamics
This is a repository copy of Alite calcium sulfoaluminate cement: chemistry and thermodynamics. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/144183/ Version: Accepted Version Article: Hanein, T. orcid.org/0000-0002-3009-703X, Duvallet, T.Y., Jewell, R.B. et al. (5 more authors) (2019) Alite calcium sulfoaluminate cement: chemistry and thermodynamics. Advances in Cement Research, 31 (3). pp. 94-105. ISSN 0951-7197 https://doi.org/10.1680/jadcr.18.00118 © 2019 ICE Publishing. This is an author produced version of a paper subsequently published in Advances in Cement Research. Uploaded in accordance with the publisher's self-archiving policy. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Abstract: Calcium sulfoaluminate (C$A) cement is a binder of increasing interest to the cement industry and as such is undergoing rapid development. Current formulations do not contain alite; however, it can be shown that hybrid C$A-alite cements can combine the favourable characteristics of Portland cement with those of C$A cement while also having a lower carbon footprint than the current generation of Portland cement clinkers. -

Cementing Qualities of the Calcium Aluminates
DEPARTMENT OF COMMERCE Technologic Papers of THE Bureau of Standards S. W. STRATTON, Director No. 19 7 CEMENTING QUALITIES OF THE CALCIUM ALUMINATES BY P. H. BATES, Chemist Bureau of Standards SEPTEMBER 27, 1921 PRICE, 10 CENTS Sold only by the Superintendent of Documents, Government Printing Office Washington, D. C. WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CEMENTING QUALITIES OF THE CALCIUM ALBUMI- NATES Bv P. H. Bates ABSTRACT four calcium aluininates (3CaO.Al 5Ca0.3Al , CaO.Al The 2 3 , 2 3 2 3 , 3Ca0.5Al 2 3 ) have been made in a pure condition and their cementing qualities determined. The first two reacted so energetically with water that too rapid set resulted to make them usable commercially. The last two set more slowly and developed very high strengths at early periods. These two alurninates high in alumina were later made in a pure and impure condition in larger quantities in a rotary kiln and concrete was made from the resulting ground clinker. A 1:1.5:5.5 gravel concrete developed in 24 hours as high strength as a similarly proportioned Portland cement concrete would have developed in 28 days. Recent investigations * have shown that lime can combine with alumina only in the following molecular proportions: 3CaO. A1 5Ca0.3Al , CaO.Al 3Ca0.5Al . These investiga- 2 3 , 2 3 2 3 , 2 3 tions also show that the tricalcium aluminate is the only aluminate present in Portland cement of normal composition and normal properties. Later these results were confirmed by work carried on at this Bureau and published as Technologic Paper No. -

Reactions Accompanying Stabilization of Clay with Cement A
Reactions Accompanying Stabilization Of Clay with Cement A. HERZOG, Senior Lecturer in Civil Engineering, University of New South Wales, Newcastle, Australia, and J. K. :MITCHELL, Assistant Professor of Civil Engineering, and Assistant Research Engineer, Institute of Transportation and Traffic Engineering, University of California, Berkeley This paper reports the results of an investigation aimed at de lineating the nature of the reactions accompanying the stabili zation of clay with portland cement. Consideration of the nature of cement hydration, the physico-chemical character istics of clays and lime-clay interaction leads to the hypothesis that during the hydration of a clay-cement mixture, hydrolysis and hydration of cement could be regarded as primary reac tions which form usual cement hydration products, increase the pH, and liberate lime. The high pH and Ca(OHh concen tration could initiate attack of the clay particles and also cause breakdown of amorphous silica and alumina which then could combine with calcium to form secondary cementitious material. A clay-cement skeleton and a clay matrix are likely. Mechanical, X-ray diffraction, and chemical tests on kao linite and montmorillonite stabilized with portland cement and with pure tricalcium silicate (C3S), the major strength-producing compound in portland cement, gave results in agreement with this hypothesis. Compacted clay-cement mixtures were cured at 100 percent relative humidity and at 60 C (to accelerate hardening) and studied at different times after molding. X-ray analyses showed that calcium hydroxide was formed in hydrat ing clay-cement, but was rapidly used up in reaction with the clay. Minor alteration of the kaolinite-cement X-ray pattern and marked alteration of the montmorillonite-cement X-ray pattern were indicated after curing periods of 12 weeks, sug gesting clay mineral structure breakdown and/or interaction with cement at particle surfaces.