Standard X-Ray Diffraction Powder Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG
Central European Journal of Energetic Materials ISSN 1733-7178; e-ISSN 2353-1843 Copyright © 2018 Institute of Industrial Organic Chemistry, Poland Cent. Eur. J. Energ. Mater. 2018, 15(1): 115-130; DOI: 10.22211/cejem/78089 Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG Jing-yuan Zhang, Xue-yong Guo,* Qing-jie Jiao, Hong-lei Zhang, Hang Li State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China * E-mail: [email protected] Abstract: The thermal decomposition characteristics of CL-20, potassium perchlorate (KP), lithium perchlorate (LP), a CL-20/KP mixture, and a CL-20/LP mixture were studied using thermogravimetry-differential scanning calorimetry (TG-DSC). The DSC curves for KP exhibited three endothermic peaks and one exothermic peak. The first two endothermic peaks correspond to the rhombic- cubic transition and the fusion of KP, respectively, the third indicates the fusion of KCl, while the exothermic peak is attributed to the decomposition of KP. The DSC curves obtained from LP showed four endothermic peaks and one exothermic peak. The first two endothermic peaks indicate the loss of adsorbed water and water of crystallization, while the third and fourth are associated with the fusion of LP and LiCl, respectively; the exothermic peak is due to the decomposition of LP. The presence of KP had little effect on the thermal decomposition of CL-20 while the addition of LP increased the temperature at which CL-20 exhibits an exothermic peak. In addition, the thermal decomposition of LP appeared to be catalyzed by the presence of CL-20. -

Breakthrough Chemistry Simulations for Lithium Processing Contents
think simulation | getting the chemistry right Breakthrough chemistry simulations for lithium processing Using simulation to maximize your investment return in lithium extraction and processing Contents Introduction ............................................................................................................................................ 2 Process simulation deficiencies ................................................................................................................ 2 OLI Systems electrolyte thermodynamics .................................................................................................2 OLI Systems lithium initiative .................................................................................................................... 2 Lithium phase 1 and potash chemistry is complete ................................................................................... 2 Fundamental sulfate – chloride systems .............................................................................................. 3 Fundamental hydroxide and carbonate systems .................................................................................. 3 Lithium in acid environments for processing and recycling ................................................................... 3 Lithium borate systems for Li production ............................................................................................. 4 Systems related to Li hydrometallurgical processing, purifications and recycling .................................. -
Evaluation of Aqueous Systems
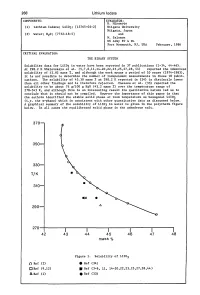
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Handbook on the Physics and Chemistry of Rare Earths Volume 9 Elsevier, 1987
Handbook on the Physics and Chemistry of Rare Earths volume 9 Elsevier, 1987 Edited by: Karl A. Gschneidner, Jr. and LeRoy Eyring ISBN: 978-0-444-87045-2 by kmno4 Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner, Jr. and L. Eyring © Elsevier Science Publishers B.V., 1987 PREFACE Karl A. GSCHNEIDNER, Jr., and LeRoy EYRING o These elements perplex us in our rearches [sic], baffle us in our speculations, and haunt us in our very dreams. They stretch like an unknown sea before us- mocking, mystifying, and murmuring strange revelations and possibilities. Sir William Crookes (February 16, 1887) There are those who feel that the rare earth elements are destined to play an even greater role in our "high-tech" society in the future than they have in the past. This judgement is based upon the trend of increasing applications resulting from the electronic structures of these materials that lead to their unusual optical, magnetic, electrical and chemical properties so adaptable to the demands now being placed on materials. The "Handbook" seeks to provide topical reviews and compilations of critically reviewed data to aid in the design and fabrication of the required new materials. Four additional chapters in this tradition are the contents of this volume. The development of lasers containing rare earth species sparked an interest in glasses containing these elements. Reisfeld and J0rgensen discuss excited-state phenomena in vitreous rare-earth-containing substances. The description of the chemistry and crystal chemistry of complex inorganic compounds of the rare earths is continued by Niinist6 and Leskelfi from the previous volume. -

Polyorganosiloxanes: Molecular Nanoparticles, Nanocomposites and Interfaces
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses November 2017 POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES Daniel H. Flagg University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Materials Chemistry Commons, Polymer and Organic Materials Commons, and the Polymer Chemistry Commons Recommended Citation Flagg, Daniel H., "POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES" (2017). Doctoral Dissertations. 1080. https://doi.org/10.7275/10575940.0 https://scholarworks.umass.edu/dissertations_2/1080 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the degree requirements for the degree of DOCTOR OF PHILOSOPHY September 2017 Polymer Science and Engineering © Copyright by Daniel H. Flagg 2017 All Rights Reserved POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Approved as to style and content by: Thomas J. McCarthy, Chair E. Bryan Coughlin, Member John Klier, Member E. Bryan Coughlin, Head, PS&E To John Null ACKNOWLEDGEMENTS There are countless individuals that I need to thank and acknowledge for getting me to where I am today. I could not have done it alone and would be a much different person if it were not for the support of my advisors, friends and family. -

The Minerals and Rocks of the Earth 5A: the Minerals- Special Mineralogy
Lesson 5 cont’d: The Minerals and Rocks of the Earth 5a: The minerals- special mineralogy A. M. C. Şengör In the previous lectures concerning the materials of the earth, we studied the most important silicates. We did so, because they make up more than 80% of our planet. We said, if we know them, we know much about our planet. However, on the surface or near-surface areas of the earth 75% is covered by sedimentary rocks, almost 1/3 of which are not silicates. These are the carbonate rocks such as limestones, dolomites (Americans call them dolostones, which is inappropriate, because dolomite is the name of a person {Dolomieu}, after which the mineral dolomite, the rock dolomite and the Dolomite Mountains in Italy have been named; it is like calling the Dolomite Mountains Dolo Mountains!). Another important category of rocks, including parts of the carbonates, are the evaporites including halides and sulfates. So we need to look at the minerals forming these rocks too. Some of the iron oxides are important, because they are magnetic and impart magnetic properties on rocks. Some hydroxides are important weathering products. This final part of Lesson 5 will be devoted to a description of the most important of the carbonate, sulfate, halide and the iron oxide minerals, although they play a very little rôle in the total earth volume. Despite that, they play a critical rôle on the surface of the earth and some of them are also major climate controllers. The carbonate minerals are those containing the carbonate ion -2 CO3 The are divided into the following classes: 1. -

Cobalt Mineral Ecology
American Mineralogist, Volume 102, pages 108–116, 2017 Cobalt mineral ecology ROBERT M. HAZEN1,*, GRETHE HYSTAD2, JOSHUA J. GOLDEN3, DANIEL R. HUMMER1, CHAO LIU1, ROBERT T. DOWNS3, SHAUNNA M. MORRISON3, JOLYON RALPH4, AND EDWARD S. GREW5 1Geophysical Laboratory, Carnegie Institution, 5251 Broad Branch Road NW, Washington, D.C. 20015, U.S.A. 2Department of Mathematics, Computer Science, and Statistics, Purdue University Northwest, Hammond, Indiana 46323, U.S.A. 3Department of Geosciences, University of Arizona, 1040 East 4th Street, Tucson, Arizona 85721-0077, U.S.A. 4Mindat.org, 128 Mullards Close, Mitcham, Surrey CR4 4FD, U.K. 5School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. ABSTRACT Minerals containing cobalt as an essential element display systematic trends in their diversity and distribution. We employ data for 66 approved Co mineral species (as tabulated by the official mineral list of the International Mineralogical Association, http://rruff.info/ima, as of 1 March 2016), represent- ing 3554 mineral species-locality pairs (www.mindat.org and other sources, as of 1 March 2016). We find that cobalt-containing mineral species, for which 20% are known at only one locality and more than half are known from five or fewer localities, conform to a Large Number of Rare Events (LNRE) distribution. Our model predicts that at least 81 Co minerals exist in Earth’s crust today, indicating that at least 15 species have yet to be discovered—a minimum estimate because it assumes that new minerals will be found only using the same methods as in the past. Numerous additional cobalt miner- als likely await discovery using micro-analytical methods. -

Inorganic Syntheses
INORGANIC SYNTHESES Volume 27 .................... ................ Board of Directors JOHN P. FACKLER, JR. Texas A&M University BODlE E. DOUGLAS University of Pittsburgh SMITH L. HOLT, JR. Oklahoma State Uniuersity JAY H. WORRELL University of South Florida RUSSELL N. GRIMES University of Virginia ROBERT J. ANGELIC1 Iowa State University Future Volumes 28 ROBERT J. ANGELIC1 Iowa State University 29 RUSSELL N. GRIMES University of Virginia 30 LEONARD V. INTERRANTE Rensselaer Polytechnic Institute 31 ALLEN H. COWLEY University of Texas, Austin 32 MARCETTA Y. DARENSBOURG Texas A&M University International Associates MARTIN A. BENNETT Australian National University, Canberra FAUSTO CALDERAZZO University of Pisa E. 0. FISCHER Technical University. Munich JACK LEWIS Cambridge University LAMBERTO MALATESTA University of Milan RENE POILBLANC University of Toulouse HERBERT W. ROESKY University of Gottingen F. G. A. STONE University of Bristol GEOFFREY WILKINSON Imperial College of Science and Technology. London AKlO YAMAMOTO Tokyo Institute 01 Technology. Yokohama Editor-in-Chief ALVIN P. GINSBERG INORGANIC SYNTHESES Volume 27 A Wiley-Interscience Publication JOHN WILEY & SONS New York Chichester Brisbane Toronto Singapore A NOTE TO THE READER This book has been electronically reproduced from digital idormation stored at John Wiley h Sons, Inc. We are phased that the use of this new technology will enable us to keep works of enduring scholarly value in print as long as there is a reasonable demand for them. The content of this book is identical to previous printings. Published by John Wiley & Sons, Inc. Copyright $? 1990 Inorganic Syntheses, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Section 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. -

(12) United States Patent (10) Patent No.: US 6,358,747 B1 Condit Et Al
USOO6358747B1 (12) United States Patent (10) Patent No.: US 6,358,747 B1 Condit et al. (45) Date of Patent: Mar. 19, 2002 (54) METHOD AND APPARATUS FOR D. S. Gaibakyan etal, J. Anal. Chem. 1970, 25, 2056–2059.* QUANTIFYING MOLYBDATE IN BRINES A. M. Kiememeljet al, Anal. Chem. 1976, 48,575-578.* (75) Inventors: David A. Condit, Avon; Mark R. H. Llambias et al, Chem. Abstr. 1978, 88, abstract 53021f.* Jaworowski, Glastonbury; Xia Tang, West Hartford, all of CT (US) Lis et al., Journal of Alloys and Compounds 303-304, 132-136, 2000.* (73) ASSignee: sayier Corporation, Farmington, CT Water Analysis Handbood, HACH Company, Loveland, Colorado, “Molybdenum, Molybdate', pp. 636. (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 * cited by examiner U.S.C. 154(b) by 0 days. (21) Appl. No.: 09/324,376 Primary Examiner Arlen Soderquist y - - - 9 (22) Filed: Jun. 2, 1999 (57) ABSTRACT (51) Int. Cl. ................................................ G01N 33/20 Amethod and apparatus are provided for quantifying molyb (52) U.S. Cl. ............................. 436/83; 422/61; 436/73; date corrosion inhibitor concentrations in lithium halide 436/166 brines of absorption refrigeration Systems. This permits (58) Field of Search ............................ 436/73, 83, 166; monitoring and control of the inhibitor level. A reagent is 422/61 chosen for reacting with the molybdate in the brine to provide a readily identifiable characteristic color, the inten (56) References Cited sity of which is a function and measure of the molybdate U.S. PATENT DOCUMENTS concentration. The reagent is an acidified reducing agent a which reacts to provide a significant characteristic color 5,106,581. -

Amélioration De La Maîtrise Des Ressources Françaises En Tungstène Improvement in Control of French Tungsten Resources BRGM
^ **' Amélioration de la maîtrise des ressources françaises en tungstène Improvement in control of french tungsten resources BRGM Rapport final M. SAVE M. PASTOR R 30346 Diffusion : MIN DAM 90 DG 1 ex page de garde, résumé, table mat., conclusions January 1990 LOG/D 1 ex non relié SGN/DIG 2 ex DS (M. Sustrac) 1 ex DAM/0P4 (M. Dumas) 1 ex DAM/MP (M. Snoep) 1 ex DAM/MIN : MM. Save 1 ex Morizot 1 ex Hau/Ollivier 1 ex chrono A.F.M.E. (M. Marcé) 6 ex CERMeP (M. Pastor) 1 ex IRCHA (Mme Presvot) 1 ex BUREAU DE RECHERCHES GÉOLOGIQUES ET MINIÈRES DIRECTION DES Activités minières Département Minéralurgie B.P. 6009 - 45060 ORLÉANS CEDEX 2 - France -Tél. : (33) 38.64.34.34 AMELIORATION DE LA MAITRISE DES RESSOURCES FRANÇAISES EN TUNGSTENE Responsable Scientifique : G. MORIZOT N* de contrat A.F.M.E. : 7.02.0035 Objet : Amélioration de la maîtrise de ressources françaises en tungstène Date de notification : 30/12/1987 Durée du contrat : 32 mois Montant du contrat : 1 600 000 F.F. H.T. Responsable A.F.M.E. : M. BEUTIN Service Industrie - Matières Premières RAPPORT CONFIDENTIEL BUREAU DE RECHERCHES GÉOLOGIQUES ET MINIÈRES DIRECTION DES ACTIVITÉS MINIERES Département Minêmlurqie B.P. G003 • 4E0H0 OKLEANS ŒDEX 2 - n.inc.* - îi'l.: (13) 23 ru IMJ.I R E S U M E Un programme général visant à améliorer la maîtrise des ressources françaises en tungstène a été entrepris par le B.R.G.M. et la Société EUROTUNGSTENE POUDRES (E.T.P.). Les études ont débuté le 1er octobre 1985 dans le cadre d'une première convention A.F.M.E. -

The Origins of Color in Minerals Four Distinct Physical Theories
American Mineralogist, Volume 63. pages 219-229, 1978 The origins of color in minerals KURT NASSAU Bell Laboratories Murray Hill, New Jersey 07974 Abstract Four formalisms are outlined. Crystal field theory explains the color as well as the fluores- cence in transition-metal-containing minerals such as azurite and ruby. The trap concept, as part of crystal field theory, explains the varying stability of electron and hole color centers with respect to light or heat bleaching, as well as phenomena such as thermoluminescence. The molecular orbital formalism explains the color of charge transfer minerals such as blue sapphire and crocoite involving metals, as well as the nonmetal-involving colors in lazurite, graphite and organically colored minerals. Band theory explains the colors of metallic minerals; the color range black-red-orange- yellow-colorless in minerals such as galena, proustite, greenockite, diamond, as well as the impurity-caused yellow and blue colors in diamond. Lastly, there are the well-known pseudo- chromatic colors explained by physical optics involving dispersion, scattering, interference, and diffraction. Introduction The approach here used is tutorial in nature and references are given for further reading or, in some Four distinct physical theories (formalisms) are instances, for specific examples. Color illustrations of required for complete coverage in the processes by some of the principles involved have been published which intrinsic constituents, impurities, defects, and in an earlier less technical version (Nassau, 1975a). specific structures produce the visual effects we desig- Specific examples are given where the cause of the nate as color. All four are necessary in that each color is reasonably well established, although reinter- provides insights which the others do not when ap- pretations continue to appear even in materials, such plied to specific situations.