Vaccination Certificate Information Version: 21 July 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Dynamics of a Vaccination Model for Infectious Diseases with Asymptomatic Carriers
MATHEMATICAL BIOSCIENCES doi:10.3934/mbe.2016019 AND ENGINEERING Volume 13, Number 4, August 2016 pp. 813{840 GLOBAL DYNAMICS OF A VACCINATION MODEL FOR INFECTIOUS DISEASES WITH ASYMPTOMATIC CARRIERS Martin Luther Mann Manyombe1;2 and Joseph Mbang1;2 Department of Mathematics, Faculty of Science University of Yaounde 1, P.O. Box 812 Yaounde, Cameroon 1;2;3; Jean Lubuma and Berge Tsanou ∗ Department of Mathematics and Applied Mathematics University of Pretoria, Pretoria 0002, South Africa (Communicated by Abba Gumel) Abstract. In this paper, an epidemic model is investigated for infectious dis- eases that can be transmitted through both the infectious individuals and the asymptomatic carriers (i.e., infected individuals who are contagious but do not show any disease symptoms). We propose a dose-structured vaccination model with multiple transmission pathways. Based on the range of the explic- itly computed basic reproduction number, we prove the global stability of the disease-free when this threshold number is less or equal to the unity. Moreover, whenever it is greater than one, the existence of the unique endemic equilibrium is shown and its global stability is established for the case where the changes of displaying the disease symptoms are independent of the vulnerable classes. Further, the model is shown to exhibit a transcritical bifurcation with the unit basic reproduction number being the bifurcation parameter. The impacts of the asymptomatic carriers and the effectiveness of vaccination on the disease transmission are discussed through through the local and the global sensitivity analyses of the basic reproduction number. Finally, a case study of hepatitis B virus disease (HBV) is considered, with the numerical simulations presented to support the analytical results. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -
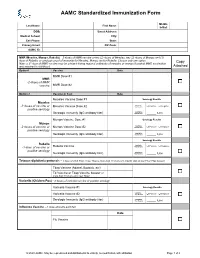
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

The Mississippi Covid-19 Vaccine Confidence Survey: Population Results Report
MAY 2021 THE MISSISSIPPI COVID-19 VACCINE CONFIDENCE SURVEY: POPULATION RESULTS Report A collaborative population-based study The Mississippi Community Engagement Alliance Against COVID-19 Disparities (CEAL) Team The Mississippi State Department of Health: Office of Preventive Health and Health Equity EXECUTIVE SUMMARY Since the Spring of 2020, the Novel 2019 Office of Preventive Health and Health Coronavirus (COVID-19) Pandemic has Equity (OPHHE) disseminated a statewide impacted Mississippians of every race, vaccine confidence survey beginning end ethnicity, age, gender, and income bracket. of December 2020 and collecting data Unfortunately, it has disproportionately until March 2021. The survey is intended to impacted Mississippians of color, the elderly, be representative of Mississippians, with and those living with chronic disease. For intentional efforts invested to reach lower most of the past year, the State has worked income and rural Mississippi populations, as to protect its population through preventive well as the state’s Black, Hispanic (Latino/ measures such as social distancing and Latinx), Asian (including the Vietnamese personal protective equipment. However, population of the Gulf Coast), and Native with the release of COVID-19 Vaccines to American/Choctaw communities. The sur- the public, the population of Mississippi has vey was administered in three languages- the opportunity to embrace a long-term English, Spanish, and Vietnamese- through a solution to COVID-19. That is, if Mississippians mixed-modal survey effort, including: web- are willing to receive the vaccine. To based, paper-based, and verbal-oratory assess Mississippians’ COVID-19 Vaccine administration. All targeted populations confidence, the Mississippi Community were ultimately reached and are represented Engagement Alliance Against COVID-19 in the over 11,000 completed responses Disparities (CEAL) Team and the Mississippi from all 82 of Mississippi’s counties. -

Parental Attitudes About Sexually Transmitted Infection Vaccination for Their Adolescent Children
ARTICLE Parental Attitudes About Sexually Transmitted Infection Vaccination for Their Adolescent Children Gregory D. Zimet, PhD; Rose M. Mays, RN, PhD; Lynne A. Sturm, PhD; April A. Ravert, MS; Susan M. Perkins, PhD; Beth E. Juliar, MA, MS Objectives: To evaluate parental attitudes about adoles- Results: The mean vaccine scenario rating was 81.3. Sexu- cent vaccination as a function of vaccine characteristics, in- ally transmitted infection vaccines (mean, 81.3) were not cluding whether the vaccine prevented a sexually transmit- rated differently than non-STI vaccines (mean, 80.0). Con- tedinfection(STI),andtoexplorepossiblesociodemographic joint analysis indicated that severity of infection and vac- predictors of acceptability of STI vaccines. cine efficacy had the strongest influence on ratings, fol- lowed by availability of behavioral prevention. Mode of Design: Participants were 278 parents who accompa- transmission had a negligible effect on ratings. Child age nied their children (69.1% female, aged 12-17 years) to (P=.08) and sex (P=.77), parent age (P=.32) and educa- appointments at medical clinics. By using computer- tion (P=.34), insurance status (P=.08), and data collection based questionnaires, parents rated 9 hypothetical vac- site (P=.48) were not significantly associated with STI vac- cine scenarios, each of which was defined along 4 di- cine acceptability. mensions: mode of transmission (STI or non-STI), severity Conclusions: Parents were accepting of the idea of vac- of infection (curable, chronic, or fatal), vaccine efficacy cinating their adolescent children against STIs. The most (50%, 70%, or 90%), and availability of behavioral meth- salient issues were severity of infection and vaccine ef- ods for prevention (available or not available). -

Improving Vaccination Coverage Fact Sheet
Improving Vaccination Coverage Fact Sheet Improving Vaccination Coverage for Vaccine-Preventable Diseases Public confidence in immunization is critical to sustaining and increasing vaccination coverage rates and preventing outbreaks of vaccine-preventable diseases (VPDs). There is some evidence suggesting vaccination requirements that have broad reach, limited exemption criteria, and strong enforcement may help promote higher rates of vaccination coverage along with complementary actions such as monitoring VPD cases, vaccination coverage, and exemption rates; and also reporting on recent VPD outbreaks. Importation of measles into the U.S. emphasizes the importance of sustaining and increasing vaccination coverage rates to prevent outbreaks of VPDs. Measles is a highly-contagious respiratory disease caused by a virus. It spreads through the air through coughing and sneezing. Measles starts with a fever, runny nose, cough, red eyes, and sore throat, and is followed by a rash that spreads all over the body. About three out of 10 people who get measles will develop one or more complications including pneumonia, ear infections, or diarrhea. Complications are more common in children younger than age 5 and adults. Measles cases and outbreaks still occur in countries in Europe, Africa, Asia, and the Pacific. Worldwide, about 20 million people get measles each year; about 146,000 die. Each year, unvaccinated people get infected while in other countries and bring the disease into the United States and spread it to others. Since 2000, when measles was declared eliminated from the U.S., the annual number of people reported to have measles ranged from a low of 37 people in 2004 to a high of 668 people in 2014. -

Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging
International Journal of Environmental Research and Public Health Review Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging Amanda Hudson 1,2,* and William J. Montelpare 3 1 Department of Mental Health and Addiction, Health PEI, Charlottetown, PE C1C 1M3, Canada 2 Department of Health Management, University of Prince Edward Island, Charlottetown, PE C1A 4P3, Canada 3 Department of Applied Human Sciences, University of Prince Edward Island, Charlottetown, PE C1A 4P3, Canada; [email protected] * Correspondence: [email protected] Abstract: Objectives: Successful immunization programs require strategic communication to increase confidence among individuals who are vaccine-hesitant. This paper reviews research on determinants of vaccine hesitancy with the objective of informing public health responses to COVID-19. Method: A literature review was conducted using a broad search strategy. Articles were included if they were published in English and relevant to the topic of demographic and individual factors associated with vaccine hesitancy. Results and Discussion: Demographic determinants of vaccine hesitancy that emerged in the literature review were age, income, educational attainment, health literacy, rurality, and parental status. Individual difference factors included mistrust in authority, disgust sensitivity, and risk aversion. Conclusion: Meeting target immunization rates will require robust public health campaigns that speak to individuals who are vaccine-hesitant in their attitudes and behaviours. Based on the assortment of demographic and individual difference factors that contribute Citation: Hudson, A.; Montelpare, to vaccine hesitancy, public health communications must pursue a range of strategies to increase W.J. Predictors of Vaccine Hesitancy: public confidence in available COVID-19 vaccines. Implications for COVID-19 Public Health Messaging. -

Shaping the Market: Gavi's Model for Bringing the Power of Vaccines To
#GaviSeth Shaping the market: Gavi’s model for bringing the power of vaccines to the world’s poorest children Seth Berkley MD National Vaccine Advisory Committee 2 February 2015, Washington DC www.gavi.org The challenge: Reducing delays in launching new vaccines in poor countries 2 #vaccineswork Gavi: an innovative public-private partnership Building on the comparative advantages of both public and private partners 3 #vaccineswork Gavi-supported vaccination programmes: an overview Refers to the first Gavi-supported introduction of each vaccine. Vaccine Investment Strategy: Gavi’s approach to prioritsing new vaccines Category VIS Criteria • Cholera Impact on child mortality Health Impact on overall mortality • Dengue impact • Hepatitis A Impact on overall morbidity • Hepatitis B (birth dose) Epidemic potential Global or regional public health priority • Hepatitis E Herd immunity Additional • Influenza* impact Availability of alternative interventions considerations • Meningococcal CYW Socio-economic inequity • Malaria Gender inequity • Rabies Disease of regional importance • Yellow Fever** Capacity and supplier base • DTP booster GAVI market shaping potential Implementation Ease of supply chain integration • Enterovirus 71 feasibility • Mumps Ease of programmatic integration Vaccine efficacy and safety * Maternal vaccination Vaccine procurement cost Cost and value ** Additional mass campaigns In-country operational cost for money Procurement cost per event averted Gavi’s unusual development model Co-financing Market- shaping Donor base -

COVID Vaccine Faqs
DOCUMENT CREATED: 12/7/20 LAST UPDATE: 5/17/21 COVID Vaccine FAQs Why Vaccinate? What’s in the Vaccine? 1. Why should I get the COVID-19 vaccine? 2. Should I get the COVID-19 vaccine now or wait? 3. What COVID-19 vaccines are currently available? 4. What is an mRNA vaccine and what is an adenovirus vector vaccine? 5. Can the COVID-19 vaccine give me COVID? Is there live virus in the vaccine? 6. Should I worry that the vaccine was made so quickly? Were steps skipped? 7. What is the difference between Emergency Use Authorization (EUA) status and full FDA (Food and Drug Administration) approval for a vaccine? 8. Who pays for the vaccine? How Well Do the Vaccines Work? 9. Which vaccine should I get? How well do they work? Are they safe? Comparing the mRNA vaccines (Pfizer and Moderna) Comparing the J&J (Janssen) vaccine to the mRNA (Pfizer, Moderna) vaccines 10. Am I protected as soon as I receive the vaccine? Can I stop wearing a mask? 11. After vaccination, can I still spread COVID-19 to my friends and family? 12. How long will the vaccine protect me? 13. What is important to know about COVID-19 variants? 14. Were different races and ethnicities included in the vaccine trials? 15. Will getting the COVID-19 vaccine make me test positive for COVID-19 if I am tested after being vaccinated? Who Should Get Vaccinated? 16. Who should get the vaccine? Who should not? 17. I already had COVID-19. Am I supposed to get the vaccine? If so, when? 18.