Vaccine Hesitancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Executive Summary: Under the Surface: Covid-19 Vaccine Narratives, Misinformation and Data Deficits on Social Media
WWW.FIRSTDRAFTNEWS.ORG @FIRSTDRAFTNEWS Executive summary Under the surface: Covid-19 vaccine narratives, misinformation and data deficits on social media AUTHORS: RORY SMITH, SEB CUBBON AND CLAIRE WARDLE CONTRIBUTING RESEARCHERS: JACK BERKEFELD AND TOMMY SHANE WWW.FIRSTDRAFTNEWS.ORG @FIRSTDRAFTNEWS Mapping competing vaccine narratives across English, Spanish and Francophone social media This research demonstrates the complexity of the vaccine Cite this report: Smith, R., Cubbon, S. & Wardle, C. (2020). Under the information ecosystem, where a cacophony of voices and surface: Covid-19 vaccine narratives, misinformation & data deficits on narratives have coalesced to create an environment of extreme social media. First Draft. https://firstdraftnews.org/vaccine- uncertainty. Two topics are driving a large proportion of the narratives-report-summary- november-2020 current global vaccine discourse, especially around a Covid-19 vaccine: the “political and economic motives” of actors and institutions involved in vaccine development and the “safety, efficacy and necessity” concerns around vaccines. Narratives challenging the safety of vaccines have been perennial players in the online vaccine debate. Yet this research shows that narratives related to mistrust in the intentions of institutions and key figures surrounding vaccines are now driving as much of the online conversation and vaccine skepticism as safety concerns. This issue is compounded by the complexities and vulnerabilities of this information ecosystem. It is full of “data deficits” — situations where demand for information about a topic is high, but the supply of credible information is low — that are being exploited by bad actors. These data deficits complicate efforts to accurately make sense of the development of a Covid-19 vaccine and vaccines more generally. -

Program Evaluation Key Outcomes and Addressing Remaining
Vaccine 31S (2013) J73–J78 Contents lists available at ScienceDirect Vaccine jou rnal homepage: www.elsevier.com/locate/vaccine Key outcomes and addressing remaining challenges—Perspectives from a final ଝ evaluation of the China GAVI project a,1 a,1 a,1 a,1 b c Weizhong Yang , Xiaofeng Liang , Fuqiang Cui , Li Li , Stephen C. Hadler , Yvan J. Hutin , d a,∗ Mark Kane , Yu Wang a Chinese Center for Disease Control and Prevention, Beijing, China b Centers for Disease Control and Prevention, Atlanta, USA c Europe Center for Disease Control and Prevention, Stockholm, Sweden d Mercer Island, Washington, USA a r t a b i c s t l r e i n f o a c t Article history: During the China GAVI project, implemented between 2002 and 2010, more than 25 million children Received 6 June 2012 received hepatitis B vaccine with the support of project, and the vaccine proved to be safe and effective. Received in revised form 24 July 2012 With careful consideration for project savings, China and GAVI continually adjusted the budget, addi- Accepted 24 September 2012 tionally allowing the project to spend operational funds to support demonstration projects to improve timely birth dose (TBD), conduct training of EPI staff, and to monitor the project impact. Results from the final evaluation indicated the achievement of key outcomes. As a result of government co-investment, Keywords: human resources at county level engaged in hepatitis B vaccination increased from 29 per county on GAVI Project average in 2002 to 66 in 2009. All project counties funded by the GAVI project use auto-disable syringes Outcomes for hepatitis B vaccination and other vaccines. -

Vaccination Certificate Information Version: 21 July 2021
معلومات عن شهادة التطعيم، بالعربية 中文疫苗接种凭证信息 Informations sur le certificat de vaccination en FRANÇAIS Информация о сертификате вакцинации на РУССКОМ ЯЗЫКЕ Información sobre el Certificado de Vacunación en ESPAÑOL UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME VACCINATION CERTIFICATE INFORMATION VERSION: 21 JULY 2021 ABOUT THE UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME The UN is committed to ensuring the protection of its personnel. Tasked by the Secretary-General, the Department of Operational Support (DOS) is leading a coordinated, UN-system-wide effort to ensure the availability of vaccine to UN personnel, their dependents and implementing partners. The roll-out of the UN System-wide COVID-19 Vaccination Programme (the “Programme”) for UN personnel will provide a significant boost to the ability of personnel to stay and deliver on the Organization's mandates, to support beneficiaries in the communities they serve, and to contribute to our on-going work to recover better together from the pandemic. Information about the Programme is available at: https://www.un.org/en/coronavirus/vaccination ABOUT THE VACCINATION CERTIFICATE Upon administration of the requisite vaccine dosage, a certificate of vaccination is generated through the Programme’s registration platform (the “Platform”). The certificate generated by the Platform uses a standardized format, similar to the one used by the WHO in its “International Certificate of Vaccination or Prophylaxis” (Yellow) vaccination booklet. Note: This certificate may not dispense with travel restrictions put in place by the country(ies) of destination. As the nature of the pandemic continues to rapidly evolve, it is highly advisable to check the latest travel regulations. -

Global Dynamics of a Vaccination Model for Infectious Diseases with Asymptomatic Carriers
MATHEMATICAL BIOSCIENCES doi:10.3934/mbe.2016019 AND ENGINEERING Volume 13, Number 4, August 2016 pp. 813{840 GLOBAL DYNAMICS OF A VACCINATION MODEL FOR INFECTIOUS DISEASES WITH ASYMPTOMATIC CARRIERS Martin Luther Mann Manyombe1;2 and Joseph Mbang1;2 Department of Mathematics, Faculty of Science University of Yaounde 1, P.O. Box 812 Yaounde, Cameroon 1;2;3; Jean Lubuma and Berge Tsanou ∗ Department of Mathematics and Applied Mathematics University of Pretoria, Pretoria 0002, South Africa (Communicated by Abba Gumel) Abstract. In this paper, an epidemic model is investigated for infectious dis- eases that can be transmitted through both the infectious individuals and the asymptomatic carriers (i.e., infected individuals who are contagious but do not show any disease symptoms). We propose a dose-structured vaccination model with multiple transmission pathways. Based on the range of the explic- itly computed basic reproduction number, we prove the global stability of the disease-free when this threshold number is less or equal to the unity. Moreover, whenever it is greater than one, the existence of the unique endemic equilibrium is shown and its global stability is established for the case where the changes of displaying the disease symptoms are independent of the vulnerable classes. Further, the model is shown to exhibit a transcritical bifurcation with the unit basic reproduction number being the bifurcation parameter. The impacts of the asymptomatic carriers and the effectiveness of vaccination on the disease transmission are discussed through through the local and the global sensitivity analyses of the basic reproduction number. Finally, a case study of hepatitis B virus disease (HBV) is considered, with the numerical simulations presented to support the analytical results. -

REALM Research Briefing: Vaccines, Variants, and Venitlation
Briefing: Vaccines, Variants, and Ventilation A Briefing on Recent Scientific Literature Focused on SARS-CoV-2 Vaccines and Variants, Plus the Effects of Ventilation on Virus Spread Dates of Search: 01 January 2021 through 05 July 2021 Published: 22 July 2021 This document synthesizes various studies and data; however, the scientific understanding regarding COVID-19 is continuously evolving. This material is being provided for informational purposes only, and readers are encouraged to review federal, state, tribal, territorial, and local guidance. The authors, sponsors, and researchers are not liable for any damages resulting from use, misuse, or reliance upon this information, or any errors or omissions herein. INTRODUCTION Purpose of This Briefing • Access to the latest scientific research is critical as libraries, archives, and museums (LAMs) work to sustain modified operations during the continuing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. • As an emerging event, the SARS-CoV-2 pandemic continually presents new challenges and scientific questions. At present, SARS-CoV-2 vaccines and variants in the US are two critical areas of focus. The effects of ventilation-based interventions on the spread of SARS-CoV-2 are also an interest area for LAMs. This briefing provides key information and results from the latest scientific literature to help inform LAMs making decisions related to these topics. How to Use This Briefing: This briefing is intended to provide timely information about SARS-CoV-2 vaccines, variants, and ventilation to LAMs and their stakeholders. Due to the evolving nature of scientific research on these topics, the information provided here is not intended to be comprehensive or final. -

Stanley A. Plotkin, MD
55936.qxp 3/10/09 4:27 PM Page 4 Stanley A. Plotkin, MD Recipient of the 2009 Maxwell Finland Award for Scientific Achievement hysician, scientist, scholar—Stanley Plotkin Dr. Plotkin was born in New York City. His father was a has successfully juggled all three roles during commercial telegrapher. His mother, who occasionally his decades of service. filled in as an accountant, mostly stayed home with Stanley PFrom his first job following graduation from and his younger sister, Brenda. At age 15, Stanley, a student medical school as an intern at Cleveland Met- at the Bronx High School of Science, discovered what he ropolitan General Hospital to his present role as consultant wanted to do with his life. After reading the novel Arrow - to the vaccine manufacturer sanofi pasteur, Dr. Plotkin has smith by Sinclair Lewis and the nonfictional work Microbe explored the world of infectious diseases and Hunters by Paul de Kruif—two books about sci- has been actively involved in developing some entists battling diseases—Stanley set his sights of the most potent vaccines against those dis- on becoming a physician and a research scien- eases. tist. “Dr. Plotkin has been a tireless advocate for Dr. Plotkin graduated from New York Uni- the protection of humans, and children in par- versity in 1952 and obtained a medical degree ticular, from preventable infectious diseases. at Downstate Medical Center in Brooklyn. He His lifetime of work on vaccines has led to pro- was a resident in pediatrics at the Children’s found reductions in both morbidity and mortality not only Hospital of Philadelphia and at the Hospital for Sick Chil- in the United States, but throughout the world,” says Vi- dren in London. -

Immunizations
Medicine Summer 2015 | Volume 14, Number 2 encephalitis tetanus RUBELLA MUMPS meningococus pertussis ENCEPHALITIS MEASLES PERTUSSIS ROTAVIRUS meningococus MUMPS meningococus MEASLES tuberculosis RUBELLA diphtheria CHICKENPOX meningococus CHICKENPOX pneumococus meningococus rotavirus ENCEPHALITIS PNEUMOCOCUS FEVER TETANUS DIPHTHERIA TETANUS hepatitismumps POLIO encephalitis hepatitis measles pneumococus POLIO DIPHTHERIA FEVER CHICKENPOX tuberculosisRUBELLA tuberculosis diphtheria DIPHTHERIA meningococusMUMPS pneumococus encephalitis ENCEPHALITIS polio TETANUS diphtheria pertussis polio hepatitisRUBELLA meningococus ENCEPHALITIS DIPHTHERIA MEASLES YELLOW RUBELLA TETANUS measles Immunizations MEASLESpolio meningococus PERTUSSIS MEASLES RUBELLA measles tetanus rubella YELLOW tuberculosisENCEPHALITIS pneumococus CHICKENPOX RUBELLA measles What physicians rubella pertussis PNEUMOCOCUS need to know encephalitis diphtheria MUMPS meningococustetanus pneumococus measles rubella tetanus measles rotavirus tetanus rubellaPOLIOmeningococus hepatitisHEPATITISFEVER PERTUSSIS RUBELLA FEVER hepatitis FEVER HEPATITIS chickenpox poliorubella encephalitisTETANUSpertussis rubella DIPHTHERIA rotavirus tuberculosis FEVERHEPATITIS POLIO yellow DIPHTHERIAYELLOW polio pneumococus YELLOW polio ENCEPHALITIS chickenpoxrubellaRUBELLA rotavirus ENCEPHALITIS pertussis ROTAVIRUS tetanus chickenpox hepatitisFEVER encephalitis ENCEPHALITIS POLIO rotavirus ROTAVIRUS DIPHTHERIA YELLOW pertussis PNEUMOCOCUS PERTUSSIS RUBELLA polio CHICKENPOX ROTAVIRUS pneumococus CHICKENPOXCHICKENPOX -

Summary of Supportive Science Regarding Thimerosal Removal
Summary of Supportive Science Regarding Thimerosal Removal Updated December 2012 www.safeminds.org Science Summary on Mercury in Vaccines (Thimerosal Only) SafeMinds Update – December 2012 Contents ENVIRONMENTAL IMPACT ................................................................................................................................. 4 A PILOT SCALE EVALUATION OF REMOVAL OF MERCURY FROM PHARMACEUTICAL WASTEWATER USING GRANULAR ACTIVATED CARBON (CYR 2002) ................................................................................................................................................................. 4 BIODEGRADATION OF THIOMERSAL CONTAINING EFFLUENTS BY A MERCURY RESISTANT PSEUDOMONAS PUTIDA STRAIN (FORTUNATO 2005) ......................................................................................................................................................................... 4 USE OF ADSORPTION PROCESS TO REMOVE ORGANIC MERCURY THIMEROSAL FROM INDUSTRIAL PROCESS WASTEWATER (VELICU 2007)5 HUMAN & INFANT RESEARCH ............................................................................................................................ 5 IATROGENIC EXPOSURE TO MERCURY AFTER HEPATITIS B VACCINATION IN PRETERM INFANTS (STAJICH 2000) .................................. 5 MERCURY CONCENTRATIONS AND METABOLISM IN INFANTS RECEIVING VACCINES CONTAINING THIMEROSAL: A DESCRIPTIVE STUDY (PICHICHERO 2002) ...................................................................................................................................................... -

Supplemental Information and Guidance for Vaccination Providers Regarding Use of 9-Valent HPV Vaccine
Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV A 9-valent human papillomavirus (HPV) vaccine (9vHPV, Gardasil 9, Merck & Co.) was licensed for use in females and males in December 2014.1,2,3,4 The 9vHPV was the third HPV vaccine licensed in the United States by the Food and Drug Administration (FDA); the other vaccines are bivalent HPV vaccine (2vHPV, Cervarix, GlaxoSmithKline), licensed for use in females, and quadrivalent HPV vaccine (4vHPV, Gardasil, Merck & Co.), licensed for use in females and males.5 In February 2015, the Advisory Committee on Immunization Practices (ACIP) recommended 9vHPV as one of three HPV vaccines that can be used for routine vaccination of females and one of two HPV vaccines for routine vaccination of males.6 After the end of 2016, only 9vHPV will be distributed in the United States. In October 2016, ACIP updated HPV vaccination recommendations regarding dosing schedules.7 CDC now recommends two doses of HPV vaccine (0, 6–12 month schedule) for persons starting the vaccination series before the 15th birthday. Three doses of HPV vaccine (0, 1–2, 6 month schedule) continue to be recommended for persons starting the vaccination series on or after the 15th birthday and for persons with certain immunocompromising conditions. Guidance is needed for persons who started the series with 2vHPV or 4vHPV and may be completing the series with 9vHPV. The information below summarizes some of the recommendations included in ACIP Policy Notes and provides additional guidance.5-7 Information about the vaccines What are some of the similarities and differences between the three HPV vaccines? y Each of the three HPV vaccines is a noninfectious, virus-like particle (VLP) vaccine. -

UNHCR COVID-19 Global Emergency Response
GLOBAL COVID-19 EMERGENCY RESPONSE 17 February 2021 UNHCR COVID-19 Preparedness and Response Highlights COVID-19 update ■ UNHCR and Gavi, the Vaccine Alliance, signed a Memorandum of Over 46,000 Understanding (MoU) on 03 February 2020, with the overall goal of ensuring reported cases refugees and other forcibly displaced can access vaccines on par with of COVID-19 nationals. The MoU also looks at expanding coverage and quality of among forcibly displaced people immunization services, promoting equity in access and uptake of vaccines, and strengthening health systems at community and primary care level. across 103 countries ■ Jordan has become one of the world’s first countries to start COVID-19 vaccinations for refugees, including vaccinations in Za’atari refugee camp on 15 February. UNHCR has been working with the Jordanian government to increase of vaccinate refugees and provide critical health, sanitation, hygiene and logistical some 7,500 cases compared support. UNHCR appeals to all countries to follow suit and include refugees in to the previous their national vaccination drives in line with COVAX allocation principles. reporting period ■ In 2020, 39.4 million persons of concern received COVID-19 assistance (numbers as of 08 February including access to protection services, shelter, health, and education. This 2021) includes over 8.5 million individuals who received cash assistance. ■ Despite an estimated 1.44 million refugees in urgent need of resettlement globally, less than 23,000 were resettled through UNHCR last year. These are the lowest refugee resettlement numbers UNHCR has witnessed in almost two decades. The drop stems from low quotas put forward by states, as well as the impact of COVID-19, which delayed departures and programmes. -
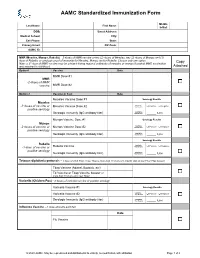
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Clinical Scholars Visit Amish Research Clinic in Lancaster County, PA by Patrick Brunner, MD Dr
Spring 2018 Center for Clinical and Translational Science e- e-NewsletterNewsletter Center News James Krueger and Marina Caskey, Representing the Nussenzweig Lab Team, Honored at Translational Science 2018 Meeting By Hospital Leadership which fulfill the translation research Dr. James Krueger received paradigm of going from bench to the Association for Clinical and bedside, have been recognized by this Translational Science (ACTS) most prestigious national recognition. Distinguished Investigator Award for his It is well deserved!” Upon receiving the groundbreaking research on psoriasis at award, Dr. Krueger noted, “This award the Translational Science 2018 meeting would not have been possible without attended by more than 1,100 people in the support of many others associated Washington, DC in April. His research with the Rockefeller CTSA enterprise: has led to a fundamental change in my lab members, the nursing staff, the paradigm for understanding the all other support departments and, pathophysiology of the disorder, and of course, hundreds of patients who this in turn has led to the development directly tested progressively better drugs of a series of novel medications that that are now used to so effectively treat precisely modulate the immune system psoriasis.” and dramatically improve the therapy of the disorder. Each year, Clinical Research Forum sponsors a competition to identify Dr. Caskey Receiving “Top Ten” Award from the “Top Ten” Clinical Research Drs. Harry Selker and Herb Pardes studies reported in the previous year. commented that “The study reflects the The competition is intense and so it very best in translational science: the is a true tribute to the novelty and careful analysis of patient phenotypes; importance of the study led by Dr.