Prenatal Testing for Chromosomal Abnormalities: an Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Oviductal Gestation in Amphibians MARVALEE H
THE JOURNAL OF EXPERIMENTAL ZOOLOGY 266394-413 (1993) Evolution of Oviductal Gestation in Amphibians MARVALEE H. WAKE Department of Integrative Biology and Museum of Vertebrate Zoology, University of California,Berkeley, California 94720 ABSTRACT Oviductal retention of developing embryos, with provision for maternal nutrition after yolk is exhausted (viviparity) and maintenance through metamorphosis, has evolved indepen- dently in each of the three living orders of amphibians, the Anura (frogs and toads), the Urodela (salamanders and newts), and the Gymnophiona (caecilians). In anurans and urodeles obligate vivi- parity is very rare (less than 1%of species); a few additional species retain the developing young, but nutrition is yolk-dependent (ovoviviparity) and, at least in salamanders, the young may be born be- fore metamorphosis is complete. However, in caecilians probably the majority of the approximately 170 species are viviparous, and none are ovoviviparous. All of the amphibians that retain their young oviductally practice internal fertilization; the mechanism is cloaca1 apposition in frogs, spermato- phore reception in salamanders, and intromission in caecilians. Internal fertilization is a necessary but not sufficient exaptation (sensu Gould and Vrba: Paleobiology 8:4-15, ’82) for viviparity. The sala- manders and all but one of the frogs that are oviductal developers live at high altitudes and are subject to rigorous climatic variables; hence, it has been suggested that cold might be a “selection pressure” for the evolution of egg retention. However, one frog and all the live-bearing caecilians are tropical low to middle elevation inhabitants, so factors other than cold are implicated in the evolu- tion of live-bearing. -

Management of Neonates Born at ≤34 6/7 Weeks' Gestation with Suspected Or Proven Early-Onset Bacterial Sepsis Karen M
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care ≤ ’ Management of Neonates Born at 34 Karen M. Puopolo, MD, PhD, FAAP, a, b William E. Benitz, MD, FAAP, c Theoklis E. Zaoutis, MD, MSCE, FAAP, a, d 6/7COMMITTEE ONWeeks FETUS AND NEWBORN, GestationCOMMITTEE ON INFECTIOUS DISEASES With Suspected or Proven Early-Onset Bacterial Sepsis Early-onset sepsis (EOS) remains a serious and often fatal illness among abstract infants born preterm, particularly among newborn infants of the lowest gestational age. Currently, most preterm infants with very low birth weight are treated empirically with antibiotics for risk of EOS, often for prolonged aDepartment of Pediatrics, Perelman School of Medicine, University periods, in the absence of a culture-confirmed infection. Retrospective of Pennsylvania, Philadelphia, Pennsylvania; bChildren’s Hospital of studies have revealed that antibiotic exposures after birth are associated Philadelphia, and dRoberts Center for Pediatric Research, Philadelphia, Pennsylvania; and cDivision of Neonatal and Developmental Medicine, with multiple subsequent poor outcomes among preterm infants, making the Department of Pediatrics, School of Medicine, Stanford University, Palo risk/benefit balance of these antibiotic treatments uncertain. Gestational Alto, California age is the strongest single predictor of EOS, and the majority of preterm This document is copyrighted and is property of the American Academy of Pediatrics and its Board of Directors. All authors have births occur in the setting of other factors associated with risk of EOS, filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process making it difficult to apply risk stratification strategies to preterm infants. -
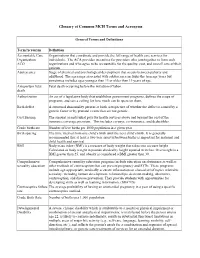
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

PSBC Obstetric Guideline: Prenatal Screening for Down Syndrome, Trisomy 18, and Open Neural Tube Defects 3 1
Perinatal Services BC Obstetric Guideline: Prenatal Screening for Down Syndrome, Trisomy 18, and Open Neural Tube Defects June 2020 Table of Contents EXECUTIVE SUMMARY � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 2 1� INTRODUCTION � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 3 SIPS, IPS, Quad, NIPT � � � � � � � � � � � � � � � � � � � � � � � � � � � � 3 Open Neural Tube Defects (ONTDs) � � � � � � � � � � � � � � � � � � � � 4 Counselling � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 4 Table 1: Summary of Prenatal Genetic Screening Tests � � � � � � � � 5 Table 2: Screening options available through the BC Prenatal Genetic Screening Program � � � � � � � � � � � � � � � � � 6 2� MANAGEMENT � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 7 3� RESOURCES � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 10 BC Prenatal Genetic Screening Program Website � � � � � � � � � � 10 Other Useful Websites � � � � � � � � � � � � � � � � � � � � � � � � � � � 10 4� BIBLIOGRAPHY � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 11 APPENDIX 1 � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 12 Risk of Down Syndrome and Other Chromosome Abnormalities in Live Births by Maternal Age � � � � � � � � � � � 12 Tel: 604-877-2121 www.bcprenatalscreening.ca APPENDIX 2 � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 13 Screen Cut-Offs and Performance of Screening Tests � � � � � � � 13 APPENDIX 3 � � � � � � � � � � � -

Genetic Testing for Reproductive Carrier Screening and Prenatal Diagnosis
Medical Coverage Policy Effective Date ............................................. 7/15/2021 Next Review Date ......................................12/15/2021 Coverage Policy Number .................................. 0514 Genetic Testing for Reproductive Carrier Screening and Prenatal Diagnosis Table of Contents Related Coverage Resources Overview ........................................................ 2 Genetics Coverage Policy ............................................ 2 Genetic Testing Collateral File Genetic Counseling ...................................... 2 Recurrent Pregnancy Loss: Diagnosis and Treatment Germline Carrier Testing for Familial Infertility Services Disease .......................................................... 3 Preimplantation Genetic Testing of an Embryo........................................................... 4 Preimplantation Genetic Testing (PGT-A) .. 5 Sequencing–Based Non-Invasive Prenatal Testing (NIPT) ............................................... 5 Invasive Prenatal Testing of a Fetus .......... 6 Germline Mutation Reproductive Genetic Testing for Recurrent Pregnancy Loss ...... 6 Germline Mutation Reproductive Genetic Testing for Infertility ..................................... 7 General Background .................................... 8 Genetic Counseling ...................................... 8 Germline Genetic Testing ............................ 8 Carrier Testing for Familial Disease ........... 8 Preimplantation Genetic Testing of an Embryo.......................................................... -

Birthweight Between 14 and 42 Weeks' Gestation
Arch Dis Child: first published as 10.1136/adc.60.5.440 on 1 May 1985. Downloaded from Archives of Disease in Childhood, 1985, 60, 440-446 Birthweight between 14 and 42 weeks' gestation D V KEEN AND R G PEARSE Jessop Hospital for Women, Sheffield SUMMARY Data representing fetal weight gain between 14 and 42 weeks' gestation are presented; firstly to provide suitable curves enabling the growth of the very immature infant to be monitored and secondly to examine the influence of the improved techniques of paediatric and obstetric assessment developed since the publication of previous studies. Data have been collected from the 57 866 livebirths in Sheffield between 1976 and 1984 and from therapeutically terminated and spontaneously aborted fetuses over the same period. It seems that preterm livebirths do not form a different population with respect to weight from the fetus still in utero, at least until the beginning of the third trimester. Previous studies have reported a bimodality of weight distribution in preterm infants at each gestational age which has been attributed to errors in gestational assessment. The pattern of distribution of weight in this study suggests that early ultrasonography and paediatric assessment techniques have exerted a considerable influence on the accuracy of gestational assessment. The mean weights of the sample differ considerably from those of the Gairdner and Pearson chart which are, therefore, considered to be inappropriate for the Sheffield population. The importance of accurate data for birthweight at It is now apparent that it is important to identify the lower gestational ages has increased with the those genuinely growth retarded infants who are improving survival of these babies. -

Prenatal Development
2 Prenatal Development Learning Objectives Conception and Genetics 2.5 What behaviors have scientists observed 2.8 How do maternal diseases and 2.1 What are the characteristics of the zygote? in fetuses? environmental hazards affect prenatal 2.1a What are the risks development? associated with assisted Problems in Prenatal Development 2.8a How has technology changed reproductive technology? 2.6 What are the effects of the major dominant, the way that health professionals 2.2 In what ways do genes influence recessive, and sex-linked diseases? manage high-risk pregnancies? development? 2.6a What techniques are used to as- 2.9 What are the potential adverse effects sess and treat problems in prena- of tobacco, alcohol, and other drugs on Development from Conception to Birth tal development? prenatal development? 2.3 What happens in each of the stages of 2.7 How do trisomies and other disorders of 2.10 What are the risks associated with legal prenatal development? the autosomes and sex chromosomes drugs, maternal diet, age, emotional 2.4 How do male and female fetuses differ? affect development? distress, and poverty? efore the advent of modern medical technology, cul- garments that are given to her by her mother. A relative ties tures devised spiritual practices that were intended to a yellow thread around the pregnant woman’s wrist as cer- B ensure a healthy pregnancy with a happy outcome. emony attendees pronounce blessings on the unborn child. For instance, godh bharan is a centuries-old Hindu cere- The purpose of the thread is to provide mother and baby mony that honors a woman’s first pregnancy. -

A Guide to Prenatal Testing
Patient Education intermountainhealthcare.org A Guide to Prenatal Testing LIVING AND LEARNING TOGETHER Most news is good news. Most babies are born without major birth defects. Early in your pregnancy, you’ll need to make decisions about prenatal testing. Prenatal tests aim to detect the risk or presence of a birth defect or serious disease in your developing baby. This guide gives you the facts you need to make decisions about testing. Spend some time with this guide. Take it home and read it carefully. At your next prenatal checkup, ask any remaining questions before making your decisions. 2 PRENATAL TESTING What’s Inside: AT A GLANCE .................................................4 TESTS: Options for screening and testing ......6 Maternal serum screening ..........................................6 Cell-free DNA (cfDNA) screening .............................8 Chorionic villus sampling (CVS) and amniocentesis 10 Carrier screening for cystic fibrosis (CF), spinal muscular atrophy (SMA), and other conditions .......................................................13 CONDITIONS: Diseases and disorders discussed in this guide .................................15 Cystic fibrosis (CF) ..................................................15 Spinal muscular atrophy (SMA) ...............................16 Turner syndrome .....................................................17 Down syndrome ......................................................18 Trisomy 18 and 13 ..................................................18 Neural tube defects (NTDs) ....................................19 -

FETAL GROWTH and DEVELOPMENT Copyright© 1995 by the South Dakota Department of Health
FETAL GROWTH AND DEVELOPMENT Copyright© 1995 by the South Dakota Department of Health. All rights reserved. The South Dakota Department of Health acknowledges Keith L. Moore, Ph.D., F.I.A.C, F.R.S.M.; T.V.N. Persaud, M.D., Ph.D., F.R.C. Path (Lond): Cynthia Barrett, M.D.; and Kathleen A. Veness-Meehan, M.D.; for their professional assis- tance in reviewing this booklet. Photos on pages 8, 9, 11, 13, 16 and 18 by Lennart Nilsson, of Sweden, A Child is Born, 1986, Dell Publishing and are used by permission. Lennart Nilsson is a pioneer in medical photography, credited with inventing numerous devices and techniques in his field. The photos used in this booklet have been published internationally in scientific periodicals and used in the popular press and television. Illustrations on pp. 5 and 6 by Drs. K.L. Moore, T.V.N. Persaud and K. Shiota, Color Atlas of Clinical Embryology, 1944, Philadelphia: W.B. Saunders, are used by permission. The South Dakota Department of Health also acknowledges the technical assis- tance of the following in development of the booklet: Gary Crum, Ph.D., Ann Kappel and Arlen Pennell of the Ohio Department of Health; Sandra Van Gerpen, M.D. M.P.H., Terry Englemann, R.N., Colleen Winter, R.N., B.S.N., and Nancy Shoup, R.N., B.S.N., of the South Dakota Department of Health; Dennis Stevens, M.D.; Virginia Johnson, M.D.; Brent Lindbloom, D.O.; Dean Madison, M.D.; Buck Williams, M.D.; Roger Martin, R.N., C.N.P., M.S.; Barbara Goddard, B.S., Ph.D.; Laurie Lippert; Vincent Rue, P.h.D.; and Representative Roger Hunt. -

Abortion at Or Over 20 Weeks' Gestation
Abortion At or Over 20 Weeks’ Gestation: Frequently Asked Questions Matthew B. Barry, Coordinator Section Research Manager April 30, 2018 Congressional Research Service 7-5700 www.crs.gov R45161 Abortion At or Over 20 Weeks’ Gestation: Frequently Asked Questions Summary Legislation at the federal and state levels seeking to limit or ban abortions in midpregnancy has focused attention on the procedure and the relatively small number of women who choose to undergo such an abortion. According to the Guttmacher Institute, about 926,200 abortions were performed in 2014; 1.3% of abortions were performed at or over 21 weeks’ gestation in 2013. A 2018 National Academies of Sciences, Engineering, and Medicine study found that most women who have abortions are unmarried (86%), are poor or low-income (75%), are under age 30 (72%), and are women of color (61%). Stages of Pregnancy and Abortion Procedures A typical, full-term pregnancy spans 40 weeks, separated into trimesters: first trimester (week 1 through week 13), second trimester (week 14 through week 27), and third trimester (week 28 through birth). Abortion in the second trimester can be performed either by using a surgical procedure or by using drugs to induce labor. According to the Centers for Disease Control and Prevention (CDC), in 2014 a surgical method was used for 98.8% of U.S. abortions at 14-15 weeks’ gestation, 98.4% at 16-17 weeks, 96.6% at 18-20 weeks, and 90.2% at 21 weeks or later. Proposed Legislation Related to Abortion Legislation in the 115th Congress, the Pain-Capable Unborn Child Protection Act (H.R. -

The Food Industry Scorecard
THE FOOD INDUSTRY SCORECARD An evaluation of food companies’ progress making—and keeping— animal welfare promises humanesociety.org/scorecard Executive summary Most of the largest U.S. food companies have publicly pledged to eliminate certain animal abuses from their supply chains. But as countless consumers have asked: are they keeping their promises? For context, the vast majority of animals in our food system live Here’s the good news: that kind of radical view is out of in dismal conditions. Mother pigs are locked in gestation crates step with traditional American values. Agribusiness may see ani- so small they can’t turn around. Egg-laying hens are crammed mals as mere machines, but consumers don’t. into cages so tightly they can’t even spread their wings. And chickens in the poultry industry are bred to grow so large, so ɠ As the American Farm Bureau reports, nearly all consumers (95%) believe farm animals should be fast they suffer from agonizing leg disorders. treated well. It wasn’t always this way. Throughout history, animals hav- en’t been forced to endure such miserable lives. (And today, ɠ The Food Marketing Institute found that animal welfare is shoppers’ second most important social issue. there are certainly farmers who don’t use these abusive prac- tices.) But as agri-culture developed into agri-business, the ɠ The food industry analytics firm Technomic concluded industry’s relationship to animals became more severe. that for American restaurant patrons, concerns about animal cruelty outweigh those regarding the “Forget the pig is an animal,” urged Hog Farm Management environment, fair trade, local sourcing and other issues. -

Information for Parents of Preterm Babies at 31 to 34 Weeks Gestation
12 Information for parents of preterm babies at 31 to 34 weeks gestation Notes and questions Information for parents of preterm babies at 31 to 34 weeks gestation Reading this booklet can answer some of the questions you may have after the birth of your preterm baby. Your baby's health care team can help by giving you the information and support you need to understand your baby's condition The staff of the Neonatal Nurseries thanks Heidi Scarfone and take part in his or her care. for the drawings in this booklet. We have a lot of information available to parents and families. In this book, If you would like more information about this artist where you see a word in bold letters, this means another handout is available. visit http://heidiscarfone.com Our materials are also available online in our Patient Education Library at www.hamiltonhealthsciences.ca Please feel free to ask questions and talk with your Obstetrician or your baby's health care team members. All your questions are welcome. © Hamilton Health Sciences, 2003 PD 4887 - 03/2013 WPC\PtEd\CH\InforParentsGest31-34Weeks-lw.doc dt/March 14, 2013 ____________________________________________________________________________ 2 11 Information for parents of preterm babies at 31 to 34 weeks gestation Information for parents of preterm babies at 31 to 34 weeks gestation What does “preterm” mean? Muscle tone and movement “Term” refers to the length of a pregnancy. “Full-term” is the length of a Preterm babies' muscles are not fully developed so they are weak. complete pregnancy - 37 to 41 weeks. “Pre-term” means “ before term”, that is At 31 weeks gestation, babies may lie quietly stretched out with some a pregnancy less than 37 weeks.