PSBC Obstetric Guideline: Prenatal Screening for Down Syndrome, Trisomy 18, and Open Neural Tube Defects 3 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prenatal Testing Options
PRENATAL TESTING OPTIONS There are many types of tests available during pregnancy. No test can detect every possible condition, however there are many tests that can provide more information. Patients may elect or decline any of these tests as they are all optional. NT Ultrasound Detailed Ultrasound SCREENING CA PNS 1st Blood Draw CA PNS 2nd Blood Draw Cell-Free DNA Screening pregnancy week 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 DIAGNOSTIC CVS Amniocentesis SCREENING TESTS Screening tests provide probabilities (odds) of having a baby with certain conditions, however screening tests cannot detect all cases of these conditions. Screening tests are performed through maternal blood and ultrasound, so there is no risk for miscarriage from these tests. Patients will receive either “screen negative” or “screen positive” results from their screening tests. When results are screen negative, these are considered low-risk for that condition, however it is not impossible that the baby has that condition. When results are screen positive, these are considered high-risk for that condition, however most women with screen positive results are carrying a healthy baby! Follow up testing is available to confirm screening results. California Prenatal Screening Program (CA PNS) (also called Sequential Integrated Screening) Combines blood draw(s) and the NT ultrasound with maternal age risk to screen for Down syndrome, trisomy 18, open neural tube defects (example: spina bifida), and Smith-Lemli-Opitz syndrome In a pregnant woman’s blood there are natural -
Chorionic Villus Sample CVS Brochure
CVS C Chorionic Villus Sampling G A A T GENETICS LABORATORIES GENETICS LABORATORIES GENETICS LABORATORIES 3/2015 ou are being asked to consider prena- tal diagnosis in your current pregnancy. Y Chorionic villus sampling (CVS) is available as a method of prenatal testing for women who are less than 13 weeks pregnant. This test can be performed earlier than amniocentesis, which is usually performed between 15 and 20 weeks, thereby offering results earlier in the pregnancy. This brochure is to provide you with some basic information on CVS. A separate brochure is avail- able which describes amniocentesis. Who should consider CVS? CVS should be considered by women age 35 or older at the time of delivery, individuals who have had a child with a chromosome abnormality, individuals who have a chromosome translocation, and couples at risk for a prenatally diagnosable genetic disease (e.g., hemophilia or sickle cell disease). CVS is not appropriate for individuals with a family history of neural tube defects (spina bifida or anencephaly). When is CVS performed? CVS is traditionally performed between 10 - 13 weeks after a woman’s last menstrual period (during the first trimester). How is CVS performed? There are two methods for obtaining chorionic villi. For many women, either method can be safely per- formed. First, an ultrasound evaluation is performed to locate the developing placenta and to date the pregnancy. Often, the placental location determines which method of CVS is more appropriate. There are certain other obstetrical considerations which may make one method preferable, including uterine anatomy and vaginal infections. TRANSCERVICAL CVS: A thin catheter (hollow tube) is inserted into the vagina and through the cervix. -

Evolution of Oviductal Gestation in Amphibians MARVALEE H
THE JOURNAL OF EXPERIMENTAL ZOOLOGY 266394-413 (1993) Evolution of Oviductal Gestation in Amphibians MARVALEE H. WAKE Department of Integrative Biology and Museum of Vertebrate Zoology, University of California,Berkeley, California 94720 ABSTRACT Oviductal retention of developing embryos, with provision for maternal nutrition after yolk is exhausted (viviparity) and maintenance through metamorphosis, has evolved indepen- dently in each of the three living orders of amphibians, the Anura (frogs and toads), the Urodela (salamanders and newts), and the Gymnophiona (caecilians). In anurans and urodeles obligate vivi- parity is very rare (less than 1%of species); a few additional species retain the developing young, but nutrition is yolk-dependent (ovoviviparity) and, at least in salamanders, the young may be born be- fore metamorphosis is complete. However, in caecilians probably the majority of the approximately 170 species are viviparous, and none are ovoviviparous. All of the amphibians that retain their young oviductally practice internal fertilization; the mechanism is cloaca1 apposition in frogs, spermato- phore reception in salamanders, and intromission in caecilians. Internal fertilization is a necessary but not sufficient exaptation (sensu Gould and Vrba: Paleobiology 8:4-15, ’82) for viviparity. The sala- manders and all but one of the frogs that are oviductal developers live at high altitudes and are subject to rigorous climatic variables; hence, it has been suggested that cold might be a “selection pressure” for the evolution of egg retention. However, one frog and all the live-bearing caecilians are tropical low to middle elevation inhabitants, so factors other than cold are implicated in the evolu- tion of live-bearing. -

Management of Neonates Born at ≤34 6/7 Weeks' Gestation with Suspected Or Proven Early-Onset Bacterial Sepsis Karen M
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care ≤ ’ Management of Neonates Born at 34 Karen M. Puopolo, MD, PhD, FAAP, a, b William E. Benitz, MD, FAAP, c Theoklis E. Zaoutis, MD, MSCE, FAAP, a, d 6/7COMMITTEE ONWeeks FETUS AND NEWBORN, GestationCOMMITTEE ON INFECTIOUS DISEASES With Suspected or Proven Early-Onset Bacterial Sepsis Early-onset sepsis (EOS) remains a serious and often fatal illness among abstract infants born preterm, particularly among newborn infants of the lowest gestational age. Currently, most preterm infants with very low birth weight are treated empirically with antibiotics for risk of EOS, often for prolonged aDepartment of Pediatrics, Perelman School of Medicine, University periods, in the absence of a culture-confirmed infection. Retrospective of Pennsylvania, Philadelphia, Pennsylvania; bChildren’s Hospital of studies have revealed that antibiotic exposures after birth are associated Philadelphia, and dRoberts Center for Pediatric Research, Philadelphia, Pennsylvania; and cDivision of Neonatal and Developmental Medicine, with multiple subsequent poor outcomes among preterm infants, making the Department of Pediatrics, School of Medicine, Stanford University, Palo risk/benefit balance of these antibiotic treatments uncertain. Gestational Alto, California age is the strongest single predictor of EOS, and the majority of preterm This document is copyrighted and is property of the American Academy of Pediatrics and its Board of Directors. All authors have births occur in the setting of other factors associated with risk of EOS, filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process making it difficult to apply risk stratification strategies to preterm infants. -

Prenatal and Preimplantation Genetic Diagnosis for Mps and Related Diseases
PRENATAL AND PREIMPLANTATION GENETIC DIAGNOSIS FOR MPS AND RELATED DISEASES Donna Bernstein, MS Amy Fisher, MS Joyce Fox, MD Families who are concerned about passing on genetic conditions to their children have several options. Two of those options are using prenatal diagnosis and preimplantation genetic diagnosis. Prenatal diagnosis is a method of testing a pregnancy to learn if it is affected with a genetic condition. Preimplantation genetic diagnosis, also called PGD, is a newer technology used to test a fertilized embryo before a pregnancy is established, utilizing in vitro fertilization (IVF). Both methods provide additional reproductive options to parents who are concerned about having a child with a genetic condition. There are two types of prenatal diagnosis; one is called amniocentesis, and the other is called CVS (chorionic villus sampling). Amniocentesis is usually performed between the fifteenth and eighteenth weeks of pregnancy. Amniocentesis involves inserting a fine needle into the uterus through the mother's abdomen and extracting a few tablespoons of amniotic fluid. Skin cells from the fetus are found in the amniotic fluid. These cells contain DNA, which can be tested to see if the fetus carries the same alterations in the genes (called mutations) that cause a genetic condition in an affected family member. If the specific mutation in the affected individual is unknown, it is possible to test the enzyme activity in the cells of the fetus. Although these methods are effective at determining whether a pregnancy is affected or not, they do not generally give information regarding the severity or the course of the condition. -

Prenatal Screening Tests: Options for Women 35 Or Older
Women who will be 35 or older on their due date are at higher risk for a group of birth defects called chromosome disorders. A screening test can give you more information about your chance to have a Prenatal Screening Tests baby with this type of birth defect. If you want a Options for women screening test during pregnancy, you can decide 35 or older which one seems right for you. How risk There are two main screening tests that look for birth defects: "State changes with age screening" and "NIPT". Either test can indicate if your pregnancy has a high or low chance for the baby to have a common chromosome disorder. Most Chance for Down syndrome pregnancies with Down syndrome and trisomy 18 can be found by starting with a screening test; however, screening tests occasionally miss these 25 year old 1 in 1250 30 year old 1 in 900 conditions. 35 year old 1 in 365 For women who will be 35 or older: 40 year old 1 in 100 • State screening detects 91-94% of babies with Down syndrome and trisomy 18 45 year old 1 in 30 • NIPT detects 98-99% of babies with Down syndrome and trisomy 18 State Screening NIPT State Screening is a more general test for birth NIPT is a more targeted screening test that looks for defects. This test estimates the risk for Down common chromosome disorders. This test estimates syndrome and trisomy 18, and can help find the risk for Down syndrome, trisomy 18, trisomy 13 pregnancies with certain physical birth defects. -

The Empire Plan SEPTEMBER 2018 REPORTING ON
The Empire Plan SEPTEMBER 2018 REPORTING ON PRENATAL CARE Every baby deserves a healthy beginning and you can take steps before your baby is even born to help ensure a great start for your infant. That’s why The Empire Plan offers mother and baby the coverage you need. When your primary coverage is The Empire Plan, the Empire Plan Future Moms Program provides you with special services. For Empire Plan enrollees and for their enrolled dependents, COBRA enrollees with their Empire Plan benefits and Young Adult Option enrollees TABLE OF CONTENTS Five Important Steps ........................................ 2 Feeding Your Baby ...........................................11 Take Action to Be Healthy; Breastfeeding and Your Early Pregnancy ................................................. 4 Empire Plan Benefits .......................................12 Prenatal Testing ................................................. 5 Choosing Your Baby’s Doctor; New Parents ......................................................13 Future Moms Program ......................................7 Extended Care: Medical Case High Risk Pregnancy Program; Management; Questions & Answers ...........14 Exercise During Pregnancy ............................ 8 Postpartum Depression .................................. 17 Your Healthy Diet During Pregnancy; Medications and Pregnancy ........................... 9 Health Care Spending Account ....................19 Skincare Products to Avoid; Resources ..........................................................20 Childbirth Education -
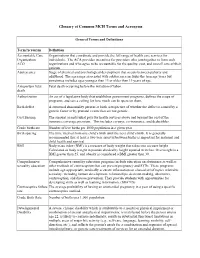
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

Genetic Testing for Reproductive Carrier Screening and Prenatal Diagnosis
Medical Coverage Policy Effective Date ............................................. 7/15/2021 Next Review Date ......................................12/15/2021 Coverage Policy Number .................................. 0514 Genetic Testing for Reproductive Carrier Screening and Prenatal Diagnosis Table of Contents Related Coverage Resources Overview ........................................................ 2 Genetics Coverage Policy ............................................ 2 Genetic Testing Collateral File Genetic Counseling ...................................... 2 Recurrent Pregnancy Loss: Diagnosis and Treatment Germline Carrier Testing for Familial Infertility Services Disease .......................................................... 3 Preimplantation Genetic Testing of an Embryo........................................................... 4 Preimplantation Genetic Testing (PGT-A) .. 5 Sequencing–Based Non-Invasive Prenatal Testing (NIPT) ............................................... 5 Invasive Prenatal Testing of a Fetus .......... 6 Germline Mutation Reproductive Genetic Testing for Recurrent Pregnancy Loss ...... 6 Germline Mutation Reproductive Genetic Testing for Infertility ..................................... 7 General Background .................................... 8 Genetic Counseling ...................................... 8 Germline Genetic Testing ............................ 8 Carrier Testing for Familial Disease ........... 8 Preimplantation Genetic Testing of an Embryo.......................................................... -

Birthweight Between 14 and 42 Weeks' Gestation
Arch Dis Child: first published as 10.1136/adc.60.5.440 on 1 May 1985. Downloaded from Archives of Disease in Childhood, 1985, 60, 440-446 Birthweight between 14 and 42 weeks' gestation D V KEEN AND R G PEARSE Jessop Hospital for Women, Sheffield SUMMARY Data representing fetal weight gain between 14 and 42 weeks' gestation are presented; firstly to provide suitable curves enabling the growth of the very immature infant to be monitored and secondly to examine the influence of the improved techniques of paediatric and obstetric assessment developed since the publication of previous studies. Data have been collected from the 57 866 livebirths in Sheffield between 1976 and 1984 and from therapeutically terminated and spontaneously aborted fetuses over the same period. It seems that preterm livebirths do not form a different population with respect to weight from the fetus still in utero, at least until the beginning of the third trimester. Previous studies have reported a bimodality of weight distribution in preterm infants at each gestational age which has been attributed to errors in gestational assessment. The pattern of distribution of weight in this study suggests that early ultrasonography and paediatric assessment techniques have exerted a considerable influence on the accuracy of gestational assessment. The mean weights of the sample differ considerably from those of the Gairdner and Pearson chart which are, therefore, considered to be inappropriate for the Sheffield population. The importance of accurate data for birthweight at It is now apparent that it is important to identify the lower gestational ages has increased with the those genuinely growth retarded infants who are improving survival of these babies. -

Prenatal Care Book – 30 Days of Your Baby’S Birth
August 2014 Prenatal NEW YORK STATE HEALTH INSURANCE PROGRAM Care (NYSHIP) for Empire Plan enrollees and for their enrolled dependents, COBRA enrollees with their Empire Plan Congratulations on your benefits and Young Adult Option enrollees pregnancy! Every baby deserves a healthy beginning. You can take steps before your baby is even born to help Five Important Steps to Having a Healthy Baby ensure a great start for your 1. Call your doctor infant. That’s why The Empire As soon as you think you are pregnant, call your doctor. Plan offers mother and baby You can do the most for your baby during the first three the coverage you need. months of pregnancy, so try to start your doctor visits as When your primary coverage soon as possible. is The Empire Plan, The The Empire Plan covers your maternity care under the Empire Plan Future Moms Medical/Surgical Program. You may choose a participating Program provides you with or non-participating provider for your maternity care. special services. Participating Provider If you choose a participating provider (obstetrician, family practice physician or certified nurse-midwife), there are no copayments for prenatal visits, delivery or your six-week checkup 3 Early Symptoms of Pregnancy after delivery. You pay only your copayment for covered services 4 Take Action to Be Healthy at participating laboratories. Exercise During Pregnancy To locate an Empire Plan participating provider or laboratory, call 5 Prenatal Testing The Empire Plan toll free at 1-877-7-NYSHIP (1-877-769-7447) 6 The Future Moms Program and select the Medical Program. -

Prenatal Development
2 Prenatal Development Learning Objectives Conception and Genetics 2.5 What behaviors have scientists observed 2.8 How do maternal diseases and 2.1 What are the characteristics of the zygote? in fetuses? environmental hazards affect prenatal 2.1a What are the risks development? associated with assisted Problems in Prenatal Development 2.8a How has technology changed reproductive technology? 2.6 What are the effects of the major dominant, the way that health professionals 2.2 In what ways do genes influence recessive, and sex-linked diseases? manage high-risk pregnancies? development? 2.6a What techniques are used to as- 2.9 What are the potential adverse effects sess and treat problems in prena- of tobacco, alcohol, and other drugs on Development from Conception to Birth tal development? prenatal development? 2.3 What happens in each of the stages of 2.7 How do trisomies and other disorders of 2.10 What are the risks associated with legal prenatal development? the autosomes and sex chromosomes drugs, maternal diet, age, emotional 2.4 How do male and female fetuses differ? affect development? distress, and poverty? efore the advent of modern medical technology, cul- garments that are given to her by her mother. A relative ties tures devised spiritual practices that were intended to a yellow thread around the pregnant woman’s wrist as cer- B ensure a healthy pregnancy with a happy outcome. emony attendees pronounce blessings on the unborn child. For instance, godh bharan is a centuries-old Hindu cere- The purpose of the thread is to provide mother and baby mony that honors a woman’s first pregnancy.