An Artist's Guide to Reversibly Color Changing Paints and Pigments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rachel Barker and Alison Bracker
Tate Papers - Beuys is Dead: Long Live Beuys! Characterising Volition,... http://www.tate.org.uk/research/tateresearch/tatepapers/05autumn/barker.htm ISSN 1753-9854 TATE’S ONLINE RESEARCH JOURNAL Beuys is Dead: Long Live Beuys! Characterising Volition, Longevity, and Decision-Making in the Work of Joseph Beuys Rachel Barker and Alison Bracker Fig.1 Joseph Beuys Felt Suit 1970 (photographed on acquisition, 1981) Felt Edition 27, no. 45 Tate Archive. Purchased by Tate 1981, de-accessioned 1995 © DACS 2005 View in Tate Collection For over four decades, artists have used organic or fugitive materials in order to instigate and interrogate processes of stasis, action, permanence, and mortality. Joseph Beuys (1921-1986), along with his contemporary, Dieter Roth (1930 –1998), pioneered this practice, probing concepts of energy, warmth, and transformation through the intelligent use of organic materials such as fat. But unlike Roth, who consistently advocated the unconstrained decomposition of his work, Beuys’ position on decay, change, and damage varied from statement to statement, and from piece to piece. As one conservator familiar with the artist’s work noted, in order to understand Beuys’ personal philosophy regarding conservation, ‘it would be necessary to read all his interviews and statements ... and even after this, one still might be restricted to speculation as to what he ... meant in this special case.’1 Unsurprisingly, museums housing Beuys sculptures and installations generally lack consistent counsel from the artist pertaining to preventive conservation, intervention, and restoration of his work. Together with codified conservation principles, artists’ statements and documentation (ideally, recorded at the time of acquisition, and at vital points in the work’s lifespan) greatly inform conservation decision- making. -

The-Cat's-Dispatch
The-Cat’s-Dispatch Walnut Ridge, Arkansas Lawrence County School District www.bobcats.k12.ar.us January 29, 2020 Vol. 45 Issue No. 4 ~ Walnut Ridge High School supplement to The Times Dispatch since 1976 ~ Augustine Soars Past 1000 Points Crowning Moments By Olivia Ford WB 2019 and Hoco 2020 Bailey Augustine has made a habit WinterBall of setting her personal bests on the basketball King and court. She leads the state in total points this Queen, season and is ranked 4th nationally in overall Chloe points. Bounds and Logan Augustine, a junior for the Lady Sain Bobcats, hit a special milestone by scoring her Homecoming 1000th career point Queen and during a victory Sweetheart against Piggott on King, December 16. Maddie On January Flippo and 13, in a 72-68 Nash Gill overtime victory against the Osceola Winter Ball 2019 Lady Seminoles, Bailey takes the court she eclipsed before a home crowd. A Winter Stroll Down Main her single game By Grady Privett scoring record by pouring in 49 points. escorted by Gavin Davis and According to Chloe Bounds and escort, Logan Sain were crowned King Kennedie Weldon, and her escort, MaxPreps, she Austin Rushing. Bailey makes every and Queen at Winter Ball 2019. leads the state in Student Council, advised basket look easy. scoring and is where they were crowned by last year’s King and Queen Nash Gill by Jerry Haynes, sponsored and ranked in the top decorated the winter wonderland. five nationally. and Katie Kersey. The annual event was held at The Studio on Saturday, Dinner was provided by H & H December 14. -

AMSA Meat Color Measurement Guidelines
AMSA Meat Color Measurement Guidelines Revised December 2012 American Meat Science Association http://www.m eatscience.org AMSA Meat Color Measurement Guidelines Revised December 2012 American Meat Science Association 201 West Springfi eld Avenue, Suite 1202 Champaign, Illinois USA 61820 800-517-2672 [email protected] http://www.m eatscience.org iii CONTENTS Technical Writing Committee .................................................................................................................... v Preface ..............................................................................................................................................................vi Section I: Introduction ................................................................................................................................. 1 Section II: Myoglobin Chemistry ............................................................................................................... 3 A. Fundamental Myoglobin Chemistry ................................................................................................................ 3 B. Dynamics of Myoglobin Redox Form Interconversions ........................................................................... 3 C. Visual, Practical Meat Color Versus Actual Pigment Chemistry ........................................................... 5 D. Factors Affecting Meat Color ............................................................................................................................... 6 E. Muscle -

01 Methods of Cooking
Food Production Foundation -II BHM -201T UNIT: 01 METHODS OF COOKING Structure 1.1 Introduction 1.2 Objectives 1.3 Heat and Cooking 1.3.1 What is heat? 1.3.2 Effect of Heat on food 1.3.3 Method of heat transfer 1.4 Methods of cooking 1.5 Moist heat Methods of Cooking 1.5.1 Boiling 1.5.2 Poaching 1.5.3 Steaming 1.5.4 Stewing 1.5.5 Braising 1.6 Dry heat Methods of Cooking 1.6.1 Baking 1.6.2 Roasting 1.6.3 Grilling 1.7 Frying 1.8 Modern Methods of cooking 1.8.1 Paper Bag (en papillotte) 1.8.2 Microwave Cooking 1.8.3 Infra-red Cooking 1.9 HACCP Standards and Professional Kitchens 1.9.1 Introduction 1.9.2 What is HACCP? 1.9.3 Food Preparation Hazard and Control Rules 1.10 Summary 1.11 Key Terms 1.12 References and Bibliography 1.13 Review Questions 1.1 Introduction This chapter deals with basic principles. You will learn about what happens to food when it is heated, about how food is cooked by different methods, and about rules of seasoning and flavouring. It is important to understand the science of food and cooking so you can successfully use these principles in the kitchen. 1.2 Objectives After reading this unit the learner will be able to understand: • Methods of heat transfer Uttarakhand Open University 1 Food Production Foundation -II BHM -201T • Effect of heat on food • Moist heat Methods of Cooking • Dry heat Methods of Cooking • Frying • Modern Methods of cooking 1.3 Heat and Cooking To cook food means to heat it in order to make certain changes in it. -

1 RON CARTER NEA Jazz Master (1998) Interviewee: Ron Carter
Funding for the Smithsonian Jazz Oral History Program NEA Jazz Master interview was provided by the National Endowment for the Arts. RON CARTER NEA Jazz Master (1998) Interviewee: Ron Carter (May 4, 1936 - ) Interviewer: Devra Hall Levy with recording engineer Ken Kimery Date: May 16, 2011 Repository: Archives Center, National Museum of American History Description: Transcript, 47 pp. Levy: It's May 16th, we're sitting in the home of Ron Carter, Jazz Master, to get the oral history for the Smithsonian Jazz Oral History Project. Ron, this is your story so please feel free to take it in any direction that you want as I go. Carter: I'll follow the questions. Levy: Okay. Well, we might as well start at the beginning. I know that you were born on May 4th of 1937 near Detroit. Tell us … you came from a large family … Carter: I'm from a family of six girls and two boys. I'm in the middle, I guess. Several of them have passed away in the intervening years. I have a sister who plays piano and flute. I have a sister who played viola, one who played violin, who passed away. I'm the only one who decided to try to make a living at this foolishness. Family of ten. Levy: That's a big family. Was it a struggle in those times financially? Was it comfortable? Carter: I think when you're that age – ten, twelve – you don't know those issues. I can assure you that my father, who had to work around the clock in those jobs given the racial tenor of the times, managed always to make sure we had plenty to eat, healthy foods. -

Thesis-1984-S782c.Pdf (5.191Mb)
-COLOR CYCLES IN WOMEN'S CLarHING FROM 1950 TO 1983, INCLUSIVE By CELIA ELAINE STALL-MEADOWS ,\\ Bachelor of Science Oklahoma State University Stillwater, Oklahoma 1981 Submitted to the Faculty of the Graduate College of the Oklahoma State University in pa:rtial fu.lf illment of the requirements MASTER OF SCIENCE ·July, 1Q84 , __ ., .. ,·-- T"'-.Q,:)uJ \(,'i8'----~i--,, J 8 v. COLOR CYCLES IN WOM.EN I S CLOTHING PROM 1950 '1'0 1983, INCLUSIVE Thesis Approved: _/~..-~ -~~-~ ""1:.-.u~,,._~,._-•=---~-•"""'""""-"""--.__,,.<..,,...,.-.,=. ..,-="""L'--.=>•=-==-"V'.,.c_..-'-=.,.-'"-~~-">""'......,....,._._ Thesis Adviser -~~--~~--- -___ dAfT---~--- ----- -·-·---- ~~.,4.tittldL "'j1~ _/!fM_.ha~-- -v-·-•- Dean of Graduate Co.1lege ii 11s9s1G I ACKNQv.lLEDGF!M.ENTS Sincere appreciation for their invaluable guidance and support is extended to Dr. Donna Branson, thesis advisor, and to the meinbers of the committee,. Dr. Tana Stufflebean and Dr. Betsy Gabb. A note of thanks is given to Dr. William Warde, statistician. The enthusiasm of my friends and family has been greatly appre ciated. Special gratitude for their love and encouragement is extended to my husband, Kendall, and our parents, Mr. and Mrs. Joe Stall and Mr. and Mrs. Alden Meadows, and my grandmother, Mrs. Russell Hale. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION. • • • • 1 Statement of the Problem. 5 Objectives. • • 5 Significance of the Study 6 Assumptions 6 Limitations • 7 II.. REVIEW OF LITERATURE • 9 Color Notation. • • 9 Classics and New Colors 12 Color as a Fashion Trend. 13 Color Preferences • • 16 Color Changes • 20 Predicting Fashion Colors • 22 Sociological and Economic Influences. • 23 Color Association of the United States. -

Word Power 60 Español Básico &
word poowweerr p Use this manual to learn basic concepts and language patterns that appear throughout the WordPower vocabularies. Sentences are included to help you and your clients practice talking with the software. Word-based Vocabularies for TouchChat, NovaChat & ChatFusion AAC Devices Copyright 2019 Nancy L. Inman, M.A.T., CCC-SLP Speech-Language Pathologist Acknowledgments Special thanks in completion of this manual and written support to Rena Carney, Meghan Conover and Lisa Timm. Nancy L. Inman Table of Contents Welcome to WordPower _________________ 5 What is WordPower? _________________ 8 Overlay Design _________________ 9 WordPower20 Simply _________________ 10 WordPower25 Touch & Scan _________________ 14 WordPower42, WordPower48, WordPower60, WordPower108 __________ 18 WordPower42 Basic & WordPower60 Basic _________________ 23 WordPower80, WordPower108 with Keyboard _________________ 29 WordPower140 Scan & Touch _________________ 34 WordPower48 Español _________________ 41 WordPower48 Español Básico _________________ 53 WordPower60 Español Básico _________________ 64 WordPower60 Español Básico Comparison Doc _________________ 76 3 Fourteen WordPower Vocabulary Files: Currently there are 14 WordPower vocabularies, which have been developed for a variety of clients, ages, and needs. They range from simple to complex, while maintaining consistency architecture and design. There are three Spanish files, and two vocabularies arranged for switch scanners, WordPower25 Touch & Scan & WordPower140 Scan & Touch. English Español WordPower20 -
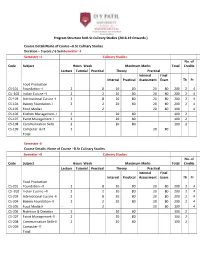
B.Sc Culinary S Duration – 3 Years / 6 Semsemester Semester
Program Structure forB.Sc Culinary Studies (2018-19 Onwards ) Course DetailsName of Course –B.Sc Culinary Studies Duration – 3 years / 6 SemSemester –I Semester –I Culinary Studies No. of Code Subject Hours Week Maximum Marks Total Credits Lecture Tutorial Practical Theory Practical Internal Final Internal Practical Assessment Exam Th Pr Food Production CS-101 Foundation –I 2 8 20 80 20 80 200 2 4 CS -102 Indian Cuisine –I 2 2 20 80 20 80 200 2 4 CS-103 International Cuisine -I 2 8 20 80 20 80 200 2 4 CS-104 Bakery Foundation- I 2 2 20 80 20 80 200 2 4 CS-105 Food Media-I - 2 20 80 100 4 CS-106 Kitchen Management- I 2 20 80 100 2 CS-107 Event Management-I 2 20 80 100 2 CS-108 Communication Skills 2 20 80 100 2 CS-109 Computer & IT 2 20 80 Total Semester -II Course Details- Name of Course –B.Sc Culinary Studies Semester –II Culinary Studies No. of Code Subject Hours Week Maximum Marks Total Credits Lecture Tutorial Practical Theory Practical Internal Final Internal Practical Assessment Exam Th Pr Food Production CS-201 Foundation –II 2 8 20 80 20 80 200 2 4 CS -202 Indian Cuisine –II 2 2 20 80 20 80 200 2 4 CS-203 International Cuisine -II 2 8 20 80 20 80 200 2 4 CS-204 Bakery Foundation- II 2 2 20 80 20 80 200 2 4 CS-205 Food Media-II - 2 20 80 100 4 CS-206 Nutrition & Dietetics 2 20 80 100 2 CS-207 Event Management-II 2 20 80 100 2 CS-208 Communication Skills-II 2 20 80 100 2 CS-209 Computer-IT Total Duration – 3 years / 6 SemSemester –III Semester –III Culinary Studies No. -

Dextrose Level and Cooking Environment Affects on Warmed-Over Flavor of Beef Top Round Roasts
DEXTROSE LEVEL AND COOKING ENVIRONMENT AFFECTS ON WARMED-OVER FLAVOR OF BEEF TOP ROUND ROASTS By KIMBERLY ANNE DUNLAVY Bachelor of Science Michigan State University East Lansing, Michigan 1990 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE May, 1993 OKLAHOMA STATE UNIVERSITY DEXTROSE LEVEL AND COOKING ENVIRONMENT AFFECfS ON WARMED-OVER FLAVOR OF BEEF TOP ROUND ROASTS Thesis Approved: ,/ The· / *~\1~ q'£ ~?lJ Dean of the Graduate College ii ACKNOWLEDGMENTS I would like to take this time to acknowledge all those people who have helped in my achieving this thesis. I would like to thank my major professor Dr. James Lamkey for taking me on as a graduate student and urging me to the completion of this feat. I would like to thank Dr. Larry Hand for getting me interested in Oklahoma State University, and for being an instructor and a friend. I would like to thank Dr. Barbara Stoecker for being a good and kind instructor, and for agreeing to complete my committee. I would be remiss if I did not thank all of the professors in the food science department for all of the help and encouragement they have given both for this specific task and in general, especially Dr. Yates, Dr. Dolezal and Dr. Rehberger. I need to thank every person who participated on my taste panel. I need to thank Dr. Horn for the use of his equipment. Thank you to all of the staff of the labs especially Chris, Siobhan, Lisa and Linda. -

Quick Guide Basic Function Navigation System Audio/Visual
Quick guide • Giving guidance for the route quickly 1 • Information to be read before operation Basic function • Setting initial settings before operation 2 • Connecting the Bluetooth® device • Operating the map screen Navigation system • Searching the map 3 • Activating the route guidance Audio/visual • Listening to the radio system • Enjoying music and video 4 Voice command system • Operating the voice command system 5 Information • Operating the weather information screen 6 Peripheral monitoring system • Checking the situation around the vehicle 7 • Operating the phone Phone 8 (Hands-free system for cellular phones) Lexus Enform • Operating the Lexus Enform 9 Index • Search alphabetically LS500_LS500h_Navi_OM50F66U_(U) 2 TABLE OF CONTENTS Introduction .......................................................5 General settings...............................53 Reading this manual.......................................7 Voice settings....................................57 Vehicle settings ............................... 58 1 Quick guide 3 Navigation system 1-1. Basic function Display and operation switches 3-1. Basic operation ................................................................12 Navigation ..........................................62 Remote Touch....................................14 Map screen operation...................64 Menu screen.......................................16 Map screen information ...............67 Status icon........................................... 18 Traffic information ...........................70 -
Copjrrightsd by Ress Basil Davis 1955 the EFFECT of PROCESSING METHODS on the COLOR of CANNED TOMATO JTJICE
Copjrrightsd by Ress Basil Davis 1955 THE EFFECT OF PROCESSING METHODS ON THE COLOR OF CANNED TOMATO JTJICE DISSERTATION Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By REES BASIL DAVIS, B.S., M.S. The Ohio State University 19511- Adviser Department of Horticulture ACKNOWLEDGEMENTS Throughout this study, the following people and organi zations have materially aided the author. In acknowledge ment of their help and the value of their association, the author wishes to express his appreciation and thanks: To Dr. Wilbur A. Gould, who has given freely and will ingly of his time, interest, guidance and advice, and es pecially whose encouragement has been an inspiration to me throughout my graduate work. To Dr. Howard D. Brown, to whom I am grateful for his guidance, advice and encouragement in this work. To Dr. Freeman S. Howlett, Chairman, Department of Horticulture, for his suggestions in the writing of this dissertation, and particularly for his guidance and encour agement throughout my graduate work. To Dr. R. C. Burrell, Department of Biochemistry, and Dr. H. H. Weiser, Department of Bacteriology, for their di rection and interest while taking my graduate work. To Mr. James 0. Mavis, who has been helpful throughout this study due to our mutual interest in color measurement problems. To the Ohio Agricultural Experiment Station and the U.S. Department of Agriculture whose cooperation in fur nishing materials, equipment and supplies made this study possible, and to the many Individuals who have materially -iii- assisted in the harvesting, processing, analysis and color evaluation of tomatoes used in this study* To my wife, whose constant interest, assistance, pa tience and inspiration have been invaluable throughout my graduate study. -

Native American Sourcebook: a Teacher's Resource on New England Native Peoples. INSTITUTION Concord Museum, MA
DOCUMENT RESUME ED 406 321 SO 028 033 AUTHOR Robinson, Barbara TITLE Native American Sourcebook: A Teacher's Resource on New England Native Peoples. INSTITUTION Concord Museum, MA. PUB DATE 88 NOTE 238p. AVAILABLE FROMConcord Museum, 200 Lexington Road, Concord, MA 01742 ($16 plus $4.95 shipping and handling). PUB TYPE Guides Classroom Use Teaching Guides (For Teacher)(052) EDRS PRICE MF01/PC10 Plus Postage. DESCRIPTORS *American Indian Culture; American Indian History; *American Indian Studies; *Archaeology; Cultural Background; Cultural Context; *Cultural Education; Elementary Secondary Education; Instructional Materials; Social Studies; Units of Study IDENTIFIERS *Algonquin (Tribe); Concord River Basin; Native Americans; *New England ABSTRACT A major aim of this source book is to provide a basic historical perspective on the Native American cultures of New England and promote a sensitive understanding of contemporary American Indian peoples. An emphasis is upon cultures which originated and/or are presently existent in the Concord River Basin. Locally found artifacts are used in the reconstruction of past life ways. This archaeological study is presented side by side with current concerns of New England Native Americans. The sourcebook is organized in six curriculum units, which include:(1) "Archaeology: People and the Land";(2) "Archaeology: Methods and Discoveries";(3) "Cultural Lifeways: Basic Needs";(4) "Cultural Lifeways: Basic Relationships"; (5) "Cultural Lifeways: Creative Expression"; and (6) "Cultural Lifeways: Native Americans Today." Background information, activities, and references are provided for each unit. Many of the lessons are written out step-by-step, but ideas and references also allow for creative adaptations. A list of material sources, resource organizations and individuals, and an index conclude the book.