Monitoring Ecosystem Quality and Function in Arid Settings of the Mojave Desert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MARCH, 1950 35 CENTS a Modern Moulting Set with Sparkling Gems of RUTILE RAINBOW DIAMONDS Is the Answer to Your Gift Problem
MARCH, 1950 35 CENTS A Modern Moulting Set With Sparkling Gems of RUTILE RAINBOW DIAMONDS Is the answer to your gift problem. One of these magnificent gems set in a lovely gold or palladium ring will be a gift that will be worn and cherished for years to come. RUTILE IS THE GEM OF THE HOUR — IT IS NOW A REALITY After years of experiments, synthetic RUTILE is now available in cut gems of breathtaking beauty. This magnificent substance has been given the name of "TITANIA". "TITANIA IS QUEEN OF ALL KNOWN GEMS" Titania gems have more fire than the diamond. Be the first in your community to own one of these beautiful new Rainbow Diamonds DEALERS INQUIRIES SOLICITED Everyone to whom you show this new gem will wish to purchase one or more of them. Our SUPERCATALOG tells you of our discount plan that will permit you to own one of these new gems at no cost to yourself. Our new SUPERCATALOG described below gives you all the facts about TITANIA GEMS and ILLUSTRATES mountings especially selected for them. An article tells the story behind this amazing discovery. The 1950 Revised Edition of Grieger's "Encyclopedia and Super Catalog of the Lapidary and Jewelry Arts" $1.00 Per Copy This is a 192-page book 9"xl2" in size. There are at least 60 pages of in- structive articles by authors of national fame. There are new articles by EMIL KRONQUIST and LOUIS WIENER on jewelry making. The 15-page article on jewelry casting by the LOST WAX METHOD using the new KERR HOBBY- CRAFT CASTING UNIT is alone worth $1.00. -

Und Audiovisuellen Archive As
International Association of Sound and Audiovisual Archives Internationale Vereinigung der Schall- und audiovisuellen Archive Association Internationale d'Archives Sonores et Audiovisuelles (I,_ '._ • e e_ • D iasa journal • Journal of the International Association of Sound and Audiovisual Archives IASA • Organie de I' Association Internationale d'Archives Sonores et Audiovisuelle IASA • Zeitschchrift der Internationalen Vereinigung der Schall- und Audiovisuellen Archive IASA Editor: Chris Clark,The British Library National Sound Archive, 96 Euston Road, London NW I 2DB, UK. Fax 44 (0)20 7412 7413, e-mail [email protected] The IASA Journal is published twice a year and is sent to all members of IASA. Applications for membership of IASA should be sent to the Secretary General (see list of officers below). The annual dues are 25GBP for individual members and IOOGBP for institutional members. Back copies of the IASA Journal from 1971 are available on application. Subscriptions to the current year's issues of the IASA Journal are also available to non-members at a cost of 35GBP I 57Euros. Le IASA Journal est publie deux fois I'an etdistribue a tous les membres. Veuillez envoyer vos demandes d'adhesion au secretaire dont vous trouverez I'adresse ci-dessous. Les cotisations annuelles sont en ce moment de 25GBP pour les membres individuels et 100GBP pour les membres institutionels. Les numeros precedentes (a partir de 1971) du IASA Journal sont disponibles sur demande. Ceux qui ne sont pas membres de I'Association peuvent obtenir un abonnement du IASA Journal pour I'annee courante au coOt de 35GBP I 57 Euro. -

Middle East Meteorology - H.M
TROPICAL METEOROLOGY- Middle East Meteorology - H.M. Hasanean MIDDLE EAST METEOROLOGY H.M. Hasanean Meteorology Department, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University Keywords: Middle East Meteorology, Arid and sub arid climate, Dust storm, Climate change, Circulation systems. Contents 1. Introduction 1.1 Middle East Definition 1.2 Overview of the Middle East Climate 2. Regional climate in the Middle East climate 2.1 Climate of Egypt 2.2 Climate of the Arabian Peninsula an Overview 2.3 Climate of Syria 2.4 Climate of Lebanon 2.5 Climate Jordan 2.6 Climate of Israel and Palestine 2.7 Climate of Cyprus 2.8 Climate of Iraq 2.9 Climate of Turkey 2.10 Climate of Iran 3. Dust storms over the Middle East 3.1 Types of Dust Storms 3.2 Synoptic Analysis of Dust Storms in the Middle East 4. Climate change over the Middle East climate 5. Climate change impacts on water resources in Middle East 6. Circulation systems affect the climate of the Middle East 6.1 Impact of the North Atlantic Oscillation (NAO) on Middle Eastern Climate 6.2 Impact of the El Nino Southern Oscillation (ENSO) on Middle East Climate 6.3 The Role of Highs Pressure (Siberian and Subtropical High Pressure) and Indian Low Pressure on Middle Eastern Climate 6.4 The roleUNESCO of Jet streams on Middle East – Climate EOLSS 7. Conclusion Acknowledgements Glossary SAMPLE CHAPTERS Bibliography Biographical Sketch Summary The Middle East is a region that spans southwestern Asia, western Asia, and northeastern Africa. Although much of the Middle East region has a Mediterranean climate type, i.e. -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

Konzept Für Lokalfauna
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Mitteilungen der Entomologischen Arbeitsgemeinschaft Salzkammergut Jahr/Year: 2004 Band/Volume: 2004 Autor(en)/Author(s): Kallies Axel, Pühringer Franz Artikel/Article: Provisional checklist of the Sesiidae of the world (Lepidoptera: Ditrysia) 1-85 ©Salzkammergut Entomologenrunde; download unter www.biologiezentrum.at Mitt.Ent.Arb.gem.Salzkammergut 4 1-85 4.12.2004 Provisional checklist of the Sesiidae of the world (Lepidoptera: Ditrysia) Franz PÜHRINGER & Axel KALLIES Abstract: A checklist of Sesiidae of the world provides 2453 names, 1562 of which are currently considered valid taxa (1 family, 2 subfamilies, 10 tribes, 149 genera, 1352 species, and 48 subspecies). Data concerning distribution, type species or type genus, designation, incorrect spelling and emendation, preoccupation and replacement names, synonyms and homonyms, nomina nuda, and rejected names are given. Several new combinations and synonyms are provided. Key words: Sesioidea, systematics, taxonomy, zoogeographic regions. Introduction: Almost 25 years have passed since HEPPNER & DUCKWORTH (1981) published their 'Classification of the Superfamily Sesioidea'. In the meantime great progress has been made in the investigation and classification of the family Sesiidae (clearwing moths). Important monographs covering the Palearctic and Nearctic regions, and partly South America or South-East Asia have been made available (EICHLIN & DUCKWORTH 1988, EICHLIN 1986, 1989, 1995b and 1998, ŠPATENKA et al. 1999, KALLIES & ARITA 2004), and numerous descriptions of new taxa as well as revisions of genera and species described by earlier authors have been published, mainly dealing with the Oriental region (ARITA & GORBUNOV 1995c, ARITA & GORBUNOV 1996b etc.). -
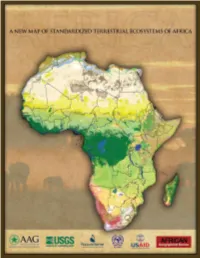
A New Map of Standardized Terrestrial Ecosystems of Africa
Major contributors to this publication include: The Association of American Geographers is a nonprofit scientific and educational society with a membership of over 10,500 individuals from more than 60 countries. AAG members are geographers and related professionals who work in the public, private, and academic sectors to advance the theory, methods, and practice of geography. This booklet is published by AAG as a special supplement to the African Geographical Review. The U.S. Geological Survey (USGS) was created in 1879 as a science agency charged with providing information and understanding to help resolve complex natural resource problems across the nation and around the world. The mission of the USGS is to provide relevant, impartial scientific information to 1) describe and understand the Earth, 2) minimize loss of life and property from natural disasters, 3) manage water, biological, energy, and mineral resources, and 4) enhance and protect our quality of life. NatureServe is an international conservation nonprofit dedicated to providing the sci- entific basis for effective conservation action. NatureServe’s network in the Americas includes more than 80 member institutions that collect and maintain a unique body of scientific knowledge about the species and ecosystems. The information products, data management tools, and biodiversity expertise that NatureServe’s scientists, technologists, and other professionals provide help meet local, national, and global conservation needs. The Regional Centre for Mapping of Resources for Development (RCMRD) was es- tablished in Nairobi, Kenya in 1975 to provide quality Geo-Information and allied Information Technology products and services in environmental and resource manage- ment for sustainable development in our member countries and beyond. -

Beobachtungen an Einer Südosthessischen Population Von Chysoesthia Drurella (Fabricius, 1775) (Lepidoptera: Gelechiidae, Gelechiinae) 181-184 Nachr
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Nachrichten des Entomologischen Vereins Apollo Jahr/Year: 2011 Band/Volume: 32 Autor(en)/Author(s): Weyh Rolf E. Artikel/Article: Beobachtungen an einer südosthessischen Population von Chysoesthia drurella (Fabricius, 1775) (Lepidoptera: Gelechiidae, Gelechiinae) 181-184 Nachr. entomol. Ver. Apollo, N. F. 32 (3/4): 181–184 (2012) 181 Beobachtungen an einer südosthessischen Population von Chysoesthia drurella (Fabricius, 1775) (Lepidoptera: Gelechiidae, Gelechiinae) Rolf E. Weyh Dipl.-Biol. Rolf E. Weyh, Somborner Straße 14, D-63579 Freigericht, Deutschland; [email protected] Zusammenfassung: Beobachtungen zu Larvalbiologie, Ver- Erscheinungszeiten und Generationenzahl puppungsweise, Überwinterungsstadium und Ge ne ra tio- nen zahl sowie zur Verbreitung der in Hessen erstmals nach- Die ersten Minen sowie einzelne fliegende Falter von C. ge wiesenen Palpenmotte Chysoesthia drurella (Fa bri ci us, drurella konnten in den Jahren 2008 bis 2011 jeweils ab 1775) (Gelechiidae) werden beschrieben, Falter und Prä ima- der dritten Maidekade festgestellt werden, das heißt, die ginalstadien farbig abgebildet. Flugzeit der ersten Faltergeneration, die bisher leider nicht direkt beobachtet werden konnte, dürfte im Mai Observations on a population of Chysoesthia drurella (Fabricius, 1775) in southeastern Hesse, Germany stattfinden. In den folgenden etwa 20 Tagen konn ten (Lepido ptera: Gelechiidae, Gelechiinae) sehr selten einzelne Falter gesichtet werden. Abstract: Information on larval biology, pupation, over win- Ab der drit ten Junidekade bis Mitte Juli traten dann te ring and number of generations as well as to distribution wie der in gro ßer Anzahl fliegende und sitzende Falter of the palpmoth Chysoesthia drurella (Fa bri ci us, 1775) auf. -

Corinna Riginos Curriculum Vitae
Corinna Riginos Curriculum Vitae Berry Biodiversity Conservation Center University of Wyoming Laramie, WY 82071 E-mail: [email protected] Phone (cell): 202-294-9731 Phone (Kenya): +254-20-2033187 EDUCATION PhD 2008 University of California, Davis: Ecology. Dissertation: Tree-grass interactions in an East African savanna: the role of wild and domestic herbivores Graduate advisor: Truman P. Young BS 2000 Brown University: Environmental Science (Magna Cum Laude) POSITIONS 2012- Berry Biodiversity Conservation Postdoctoral Fellow, University of Wyoming 2008-2012 Council on Science and Technology Postdoctoral Fellow and Lecturer, Princeton University 2000-2002 Fulbright U.S. Student Scholar, University of Stellenbosch, South Africa RESEARCH EXPERIENCE 2008-2012 Postdoctoral research: Led design and execution of studies on (a) using livestock to engineer rangelands for wildlife conservation, (b) spatial dynamics of ecosystem functioning, breakdown, and restoration in an African savanna. Additionally, developed methods and manual for pastoralist-based rangeland monitoring under contract from USAID / CARE International. Currently being used on 35,000 km2 in sub-Saharan Africa. Collaborators: Jayne Belnap, Jeff Herrick, Daniel Rubenstein, Kelly Caylor 2003-2005 Doctoral dissertation research: Led design and execution of a series of studies to test causes and consequences of increases in woody vegetation in an African savanna. Statistical modeling and field experimentation. Advisor: Truman Young 2000-2002 Fulbright fellowship: Effects of livestock grazing and disturbance on population and community ecology of succulent shrubs, Succulent Karoo, South Africa. Advisors: Suzanne J. Milton, M. Timm Hoffman 1999-2000 Honors thesis research: Maternal effects of drought stress and inbreeding on a New England forest herb. Advisors: Johanna Schmitt, M. -

Docket 07-Afc-5
DOCKET 07-AFC-5 DATE SEP 24 2008 RECD. SEP 24 2008 Ivanpah Solar Electric Generating System (ISEGS) (07-AFC-5) Supplemental Data Response, Set 1D (Responses to: Biological Resources) Submitted to the California Energy Commission Submitted by Solar Partners I, LLC; Solar Partners II, LLC; Solar Partners IV, LLC; and Solar Partners VIII, LLC September 24, 2008 With Assistance from 2485 Natomas Park Drive Suite 600 Sacramento, CA 95833 Introduction Attached are supplemental responses (Set 1D) by Solar Partners I, LLC; Solar Partners II, LLC; Solar Partners IV, LLC; and Solar Partners VIII, LLC (Applicant) to the California Energy Commission (CEC) Staff’s data requests for the Ivanpah Solar Electric Generating System (Ivanpah SEGS) Project (07-AFC-5). These data requests are the result of the workshop discussion held at Primm, Nevada on June 23, 2008.Within each discipline area, the responses are presented in alphabetical order and are numbered for tracking and reference convenience. New graphics or tables are numbered in reference to the Supplemental Data Request number. For example, if a table were used in response to Data Request AQ-1, it would be numbered Table AQ1-1. The first figure used in response to Data Request AQ-1 would be Figure AQ1-1, and so on. AFC figures or tables that have been revised have “R1” following the original number, indicating revision 1. Additional tables, figures, or documents submitted in response to a supplemental data request (supporting data, stand-alone documents such as plans, folding graphics, etc.) are found at the end of a discipline-specific section and may not be sequentially page-numbered consistently with the remainder of the document, though they may have their own internal page numbering system. -

Drought and Livestock in Semi-Arid Africa and Southwest Asia
Working Paper 117 DROUGHT AND LIVESTOCK IN SEMI-ARID AFRICA AND SOUTHWEST ASIA Roger Blench Zoë Marriage March 1999 Overseas Development Institute Portland House Stag Place London SW1E 5DP Acknowledgements The first version of this paper and the annotated bibliography was prepared as a keynote document for the FAO-sponsored Electronic Conference ‘Drought and livestock in semi-arid Africa and the Near East’, which took place between July and September 1998. The papers from the conference can be accessed at http://www.fao.org/ag/aga/agap/lps/drought1.htm. The authors are grateful to all those who took part in the conference, and the revision of this document reflects both specific comments on the text and some of the general discussion that formed part of the conference. We would like to thank Andy Catley, Maryam Fuller and Simon Mack for their observations and additional references; we hope their concerns are reflected in this revised text. This working paper is distributed by the Overseas Development Institute (ODI), an independent, non-profit policy research institute, with financial support from the Food and Agriculture Organization of the United Nations (FAO) and the Natural Resources Institute (NRI) under funding from the Department for International Development. Opinions expressed do not necessarily reflect the views of ODI, FAO, NRI or the Department for International Development. Roger Blench ([email protected]) is a Research Fellow, and Zoë Marriage (z.marriage@ odi.org.uk) is a Research Assistant, at the Overseas Development Institute. Editing, index and layout by Paul Mundy, Weizenfeld 4, 51467 Bergisch Gladbach, Germany; [email protected], http://www.netcologne.de/~nc-mundypa ISBN 0 85003 416 7 © Overseas Development Institute 1999 All rights reserved. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

Rachel Barker and Alison Bracker
Tate Papers - Beuys is Dead: Long Live Beuys! Characterising Volition,... http://www.tate.org.uk/research/tateresearch/tatepapers/05autumn/barker.htm ISSN 1753-9854 TATE’S ONLINE RESEARCH JOURNAL Beuys is Dead: Long Live Beuys! Characterising Volition, Longevity, and Decision-Making in the Work of Joseph Beuys Rachel Barker and Alison Bracker Fig.1 Joseph Beuys Felt Suit 1970 (photographed on acquisition, 1981) Felt Edition 27, no. 45 Tate Archive. Purchased by Tate 1981, de-accessioned 1995 © DACS 2005 View in Tate Collection For over four decades, artists have used organic or fugitive materials in order to instigate and interrogate processes of stasis, action, permanence, and mortality. Joseph Beuys (1921-1986), along with his contemporary, Dieter Roth (1930 –1998), pioneered this practice, probing concepts of energy, warmth, and transformation through the intelligent use of organic materials such as fat. But unlike Roth, who consistently advocated the unconstrained decomposition of his work, Beuys’ position on decay, change, and damage varied from statement to statement, and from piece to piece. As one conservator familiar with the artist’s work noted, in order to understand Beuys’ personal philosophy regarding conservation, ‘it would be necessary to read all his interviews and statements ... and even after this, one still might be restricted to speculation as to what he ... meant in this special case.’1 Unsurprisingly, museums housing Beuys sculptures and installations generally lack consistent counsel from the artist pertaining to preventive conservation, intervention, and restoration of his work. Together with codified conservation principles, artists’ statements and documentation (ideally, recorded at the time of acquisition, and at vital points in the work’s lifespan) greatly inform conservation decision- making.