View PDF Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urine Elements Resource Guide
Urine Elements Resource Guide Science + Insight doctorsdata.com Doctor’s Data, Inc. Urine Elements Resource Guide B Table of Contents Sample Report Sample Report ........................................................................................................................................................................... 1 Urine Toxic Metals Profile Introduction .................................................................................................................................................................................3 Aluminum .....................................................................................................................................................................................3 Antimony .......................................................................................................................................................................................4 Arsenic ............................................................................................................................................................................................ 4 Barium ............................................................................................................................................................................................. 5 Beryllium ........................................................................................................................................................................................5 Bismuth ......................................................................................................................................................................................... -

Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film Wte2
Lawrence Berkeley National Laboratory Recent Work Title Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2. Permalink https://escholarship.org/uc/item/1642v6qf Journal Scientific reports, 8(1) ISSN 2045-2322 Authors Asaba, Tomoya Wang, Yongjie Li, Gang et al. Publication Date 2018-04-25 DOI 10.1038/s41598-018-24736-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificreports OPEN Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2 Received: 13 December 2017 Tomoya Asaba1, Yongjie Wang 2, Gang Li 1, Ziji Xiang1, Colin Tinsman1, Lu Chen1, Accepted: 5 April 2018 Shangnan Zhou1, Songrui Zhao2, David Laleyan2, Yi Li3, Zetian Mi2 & Lu Li 1 Published: xx xx xxxx In conventional superconductors an external magnetic feld generally suppresses superconductivity. This results from a simple thermodynamic competition of the superconducting and magnetic free energies. In this study, we report the unconventional features in the superconducting epitaxial thin flm tungsten telluride (WTe2). Measuring the electrical transport properties of Molecular Beam Epitaxy (MBE) grown WTe2 thin flms with a high precision rotation stage, we map the upper critical feld Hc2 at diferent temperatures T. We observe the superconducting transition temperature Tc is enhanced by in-plane magnetic felds. The upper critical feld Hc2 is observed to establish an unconventional non- monotonic dependence on temperature. We suggest that this unconventional feature is due to the lifting of inversion symmetry, which leads to the enhancement of Hc2 in Ising superconductors. Superconductivity generally competes with magnetic felds. Based on thermodynamics, an applied magnetic feld usually suppresses superconductivity by destroying the underlying electron pairing in the superconducting state1. -

Wte2): an Atomic Layered Semimetal
The Pennsylvania State University The Graduate School Department of Materials Science and Engineering TUNGSTEN DITELLURIDE (WTE2): AN ATOMIC LAYERED SEMIMETAL A Thesis in Materials Science and Engineering by Chia-Hui Lee 2015 Chia-Hui Lee Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science December 2015 ii The thesis of Chia-Hui Lee was reviewed and approved* by the following: Joshua A. Robinson Professor of Materials Science and Engineering Thesis Advisor Thomas E. Mallouk Head of the Chemistry Department Evan Pugh University Professor of Chemistry, Physics, Biochemistry and Molecular Biology Mauricio Terrones Professor of Physics, Chemistry and Materials Science & Engineering Suzanne Mohney Professor of Materials Science and Engineering and Electrical Engineering Chair, Intercollege Graduate Degree Program in Materials Science and Engineering *Signatures are on file in the Graduate School iii ABSTRACT Tungsten ditelluride (WTe2) is a transition metal dichalcogenide (TMD) with physical and electronic properties that make it attractive for a variety of electronic applications. Although WTe2 has been studied for decades, its structure and electronic properties have only recently been correctly described. We explored WTe2 synthesis via chemical vapor transport (CVT) method for bulk crystal, and chemical vapor deposition (CVD) routes for thin film material. We employed both experimental and theoretical techniques to investigate its structural, physical and electronic properties of WTe2, and verify that WTe2 has its minimum energy configuration in a distorted 1T structure (Td structure), which results in metallic-like behavior. Our findings confirmed the metallic nature of WTe2, introduce new information about the Raman modes of Td-WTe2, and demonstrate that Td- WTe2 is readily oxidized via environmental exposure. -

View PDF Version
Nanoscale Advances View Article Online REVIEW View Journal | View Issue In-plane anisotropic electronics based on low- symmetry 2D materials: progress and prospects Cite this: Nanoscale Adv., 2020, 2,109 Siwen Zhao, a Baojuan Dong,bc Huide Wang,a Hanwen Wang,bc Yupeng Zhang, a Zheng Vitto Han*bc and Han Zhang *a Low-symmetry layered materials such as black phosphorus (BP) have been revived recently due to their high intrinsic mobility and in-plane anisotropic properties, which can be used in anisotropic electronic and optoelectronic devices. Since the anisotropic properties have a close relationship with their anisotropic structural characters, especially for materials with low-symmetry, exploring new low- symmetry layered materials and investigating their anisotropic properties have inspired numerous research efforts. In this paper, we review the recent experimental progresses on low-symmetry layered Received 4th October 2019 materials and their corresponding anisotropic electrical transport, magneto-transport, optoelectronic, Accepted 30th October 2019 thermoelectric, ferroelectric, and piezoelectric properties. The boom of new low-symmetry layered DOI: 10.1039/c9na00623k materials with high anisotropy could open up considerable possibilities for next-generation anisotropic Creative Commons Attribution 3.0 Unported Licence. rsc.li/nanoscale-advances multifunctional electronic devices. 1. Introduction energy band structure and can be regarded as a process of lowering the dimensionality of the carrier transport. Therefore, Two dimensional (2D) layered materials with strong in-plane the electrical, optical, thermal, and phonon properties of these covalent bonds and weak out-of-plane van der Waals interac- anisotropic materials are diverse along the different in-plane tions span a very broad range of solids and exhibit extraordinary crystal directions. -
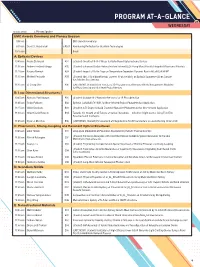
Program-At-A-Glance (PDF)
PROGRAM AT-A-GLANCE WEDNESDAY All times are EDT Plenary Speaker EMC Awards Ceremony and Plenary Session 9:00 am EMC Awards Ceremony 9:15 am David D. Awschalom PL01 Abandoning Perfection for Quantum Technologies 10:15 am Break A: Epitaxial Devices 10:45 am Rasha El-Jaroudi A01 (Student) Growth of B-III-V Alloys for GaAs-Based Optoelectronic Devices 11:00 am Andrew Frederick Briggs A02 (Student) Enhanced Double Heterostructure Infrared LEDs Using Monolithically Integrated Plasmonic Materials 11:15 am Nayana Remesh A03 (Student) Impact of Buffer Traps on Temperature-Dependent Dynamic Ron in AlGaN/GaN HEMT 11:30 am Michael Pedowitz A05 (Student) Mn+3 Rich Nanofiberous Layeredδ -phase MnO2 on Epitaxial Graphene-Silicon Carbide for Selective Gas Sensing 11:45 am Li-Chung Shih A06 (LATE NEWS, Student) Dual-Function ZTO Phototransistor Memory with Au Nanoparticles Mediated for Photo-Sensing and Multilevel Photo-Memory B: Low-Dimensional Structures I 10:45 am Nicholas Paul Morgan B01 (Student) Scalable III-V Nanowire Networks for IR Photodetection 11:00 am Rabin Pokharel B02 Epitaxial GaAsSbN (Te) NWs for Near-Infrared Region Photodetection Application 11:15 am Shisir Devakota B03 (Student) A Te Doped GaAsSb Ensemble Nanowire Photodetector for Near-Infrared Application 11:30 am Gilbert Daniel Nessim B04 Towards the Growth of 3D Forests of Carbon Nanotubes—Selective Height Control Using Thin-Film Reservoirs and Overlayers 11:45 am Dylan J. McIntyre B06 (LATE NEWS, Student) Enhancement of Integrated Cu-Ti-CNT Conductors via Joule-Heating Driven -

Large-Area and High-Quality 2D Transition Metal Telluride
Large-area and high-quality 2D transition metal telluride Jiadong Zhou1†, Fucai Liu1†, Junhao Lin2,3,4*, Xiangwei Huang5†, Juan Xia6, Bowei Zhang1, Qingsheng Zeng1, Hong Wang1, Chao Zhu1, Lin Niu1, Xuewen Wang1, Wei Fu1, Peng Yu1, Tay- Rong Chang7, Chuang-Han Hsu8,9, Di Wu8,9, Horng-Tay Jeng7,10, Yizhong Huang1, Hsin Lin8,9, , Zexiang Shen1,6,11, Changli Yang5,12, Li Lu5,12, Kazu Suenaga4, Wu Zhou2, Sokrates T. Pantelides2,3, Guangtong Liu5* and Zheng Liu1, 13,14*. 1Centre for Programmable Materials, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore 2Materials Science and Technology Division, Oak Ridge National Lab, Oak Ridge Tennessee 37831, USA 3Department of Physics and Astronomy, Vanderbilt University, Nashville, TN 37235, USA 4National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba 305-8565, Japan 5Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China 6Division of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 7Department of Physics, National Tsing Hua University, Hsinchu 30013, Taiwan 8Centre for Advanced 2D Materials and Graphene Research Centre, National University of Singapore, Singapore 117546 9Department of Physics, National University of Singapore, Singapore 117542 10Institute of Physics, Academia Sinica, Taipei 11529, Taiwan 11Centre for Disruptive Photonic Technologies, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 12Collaborative Innovation Center of Quantum Matter, Beijing 100871, China 13Centre for Micro-/Nano-electronics (NOVITAS), School of Electrical & Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore 14CINTRA CNRS/NTU/THALES, UMI 3288, Research Techno Plaza, 50 Nanyang Drive, Border X Block, Level 6, Singapore 637553, Singapore † These authors contributed equally to this work. -

The Health and Environmental Impacts of Hazardous Wastes
The health and environmental impacts of hazardous wastes IMPACT PROFILES Final report Prepared for: The Department of the Environment Prepared by: Ascend Waste and Environment Pty Ltd Date::7 June 2015 Geoff Latimer Project Number: Project # 15001AG The health and environmental impacts of hazardous wastes Project # 15001AG Date: 7 June 2015 This report has been prepared for The Department of the Environment in accordance with the terms and conditions of appointment dated 6 January 2015, and is based on the assumptions and exclusions set out in our scope of work. The report must not be reproduced in whole or in part except with the prior consent of Ascend Waste and Environment Pty Ltd and subject to inclusion of an acknowledgement of the source. Whilst reasonable attempts have been made to ensure that the contents of this report are accurate and complete at the time of writing, Ascend Waste and Environment Pty Ltd cannot accept any responsibility for any use of or reliance on the contents of this report by any third party. © Ascend Waste and Environment Pty Ltd VERSION CONTROL RECORD Document File Name Date Issued Version Author Reviewer Draft first profiles for review 11 March 2015 Rev 0 Geoff Latimer Ian Rae 15001AG_AWE_Health Env 27 April 2015 Rev0 Geoff Latimer Ian Rae Impacts draft report rev 0 15001AG_AWE_Health Env 7 June 2015 Rev0 Geoff Latimer Ian Rae Impacts final report rev 0 The health and environmental impacts of hazardous wastes Contents Page 1 Introduction 5 2 Summary report: Australia’s key hazardous waste impacts and risks -

Surface and Interface Characterization of 2D Materials: Transition Metal Dichalcogenide and Black Phosphorous
SURFACE AND INTERFACE CHARACTERIZATION OF 2D MATERIALS: TRANSITION METAL DICHALCOGENIDE AND BLACK PHOSPHOROUS by Hui Zhu APPROVED BY SUPERVISORY COMMITTEE: ___________________________________________ Dr. Robert M. Wallace, Chair ___________________________________________ Dr. Christopher L. Hinkle ___________________________________________ Dr. Jiyoung Kim ___________________________________________ Dr. Kyeongjae Cho Copyright 2017 Hui Zhu All Rights Reserved Dedicated to my husband and my parents SURFACE AND INTERFACE CHARACTERIZATION OF 2D MATERIALS: TRANSITION METAL DICHALCOGENIDE AND BLACK PHOSPHOROUS by HUI ZHU, BS, MS DISSERTATION Presented to the Faculty of The University of Texas at Dallas in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN MATERIALS SCIENCE AND ENGINEERING THE UNIVERSITY OF TEXAS AT DALLAS December 2017 ACKNOWLEDGMENTS First of all, I’d like to thank my supervisor, Dr. Robert M. Wallace, for his continuous and tremendous support of my master’s and Ph.D. research, for his patience, motivation, and immense knowledge. His diligent working attitude and enthusiasm for science establish a great standard of learning for every researcher in our group, including me. An open, friendly, and positive working environment is created, and it is my pleasure to work and study there. I would also like to acknowledge my committee professors, Dr. Jiyoung Kim, Dr. Kyeongjae Cho, and Dr. Christopher L. Hinkle for their stimulating instruction and the supportive research environment they provided. Grateful appreciation is also delivered to my colleague Mr. Qingxiao Wang and his supervisor Dr. Moon J. Kim for their extensive STEM collaborations included in this work. My acknowledgment to all my colleagues working together on 2D materials at UTD, including postdoctoral researchers: Dr. -

Bacterial Recovery and Recycling of Tellurium from Tellurium- Containing Compounds by Pseudoalteromonas Sp
Bacterial recovery and recycling of tellurium from tellurium- containing compounds by Pseudoalteromonas sp. EPR3 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Bonificio, W.D., and D.R. Clarke. 2014. “ Bacterial Recovery and Recycling of Tellurium from Tellurium-Containing Compounds by Pseudoalteromonas Sp. EPR3 .” Journal of Applied Microbiology 117 (5) (September 26): 1293–1304. doi:10.1111/jam.12629. Published Version doi:10.1111/jam.12629 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:13572100 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP 1 For submission to Journal of Applied Microbiology 2 3 Bacterial recovery and recycling of tellurium from tellurium-containing 4 compounds by Pseudoalteromonas sp. EPR3 5 William D. Bonificio #, David R. Clarke 6 7 School of Engineering and Applied Sciences, Harvard University, 29 Oxford St., 8 Cambridge, MA 02138, United States 9 10 Running Title: Bacterial recovery of tellurium 11 12 # Corresponding Author to whom inquiries should be addressed. 13 Mailing address: McKay 405, 9 Oxford St. Cambridge, MA 02138. 14 Email address: [email protected] 15 16 Keywords: Pseudoalteromonas; tellurium; tellurite; recycling; tellurium-containing 17 compounds. 1 18 ABSTRACT 19 Aims: Tellurium based devices, such as photovoltaic (PV) modules and 20 thermoelectric generators, are expected to play an increasing role in renewable energy 21 technologies. -

Intrinsic Phonon Bands in High-Quality Monolayer T' Molybdenum Ditelluride
Intrinsic Phonon Bands in High-Quality Monolayer T’ Molybdenum Ditelluride Shao-Yu Chen†, Carl H. Naylor§, Thomas Goldstein†, A.T. Charlie Johnson§, and Jun Yan†* †Department of Physics, University of Massachusetts, Amherst, Massachusetts 01003, United States §Department of Physics and Astronomy, University of Pennsylvania, Philadelphia, Pennsylvania 19104, United States *Corresponding Author: Jun Yan. Tel: (413)545-0853 Fax: (413)545-1691 E-mail: [email protected] 1 Abstract The topologically nontrivial and chemically functional distorted octahedral (T’) transition metal dichalcogenides (TMDCs) are a type of layered semimetal that has attracted significant recent attention. However, the properties of monolayer (1L) T’-TMDC, a fundamental unit of the system, is still largely unknown due to rapid sample degradation in air. Here we report that well-protected 1L CVD T'-MoTe2 exhibits sharp and robust intrinsic Raman bands, with intensities about one order of magnitude stronger than those from bulk T'-MoTe2. The high-quality samples enabled us to reveal the set of all nine even- parity zone-center optical phonons, providing reliable fingerprints for the previously elusive crystal. By performing light polarization and crystal orientation resolved scattering analysis, we can effectively distinguish the intrinsic modes from Te-metalloid-like modes A (~122 cm-1) and B (~141 cm-1) which are related to the sample degradation. Our studies offer a powerful non-destructive method for assessing sample quality and for monitoring sample degradation in situ, representing a solid advance in understanding the fundamental properties of 1L-T'-TMDCs. Keywords Transition metal dichalcogenide, monolayer T’ molybdenum ditelluride, Raman scattering, anisotropy, topological insulator, crystal quality 2 Molybdenum and tungsten based transition metal dichalcogenides (TMDCs) are a class of layered materials hosting versatile phases and polymorphs that exhibit fascinating physical, chemical and 1–5 topological properties. -

Manufacture of Cadmium Mercury Telluride
Europâisches Patenta mt ® COÏ) EuropeanEur°Pean Patent Office ® Publication number: 0 040 939 Office européen des brevets B1 ® EUROPEAN PATENT SPECIFICATION ® Date of publication of patent spécification: 02.01 .85 ® '"t. Cl.4: C 30 B 25/02, C 30 B 29/48 ® Application number: 81302178.9 (22) Dateoffiling: 15.05.81 (54) Manufacture of cadmium mercury telluride. (30) Priority: 27.05.80 GB 8017334 ® Proprietor: The Secretary of State for Defence in Her Britannic Majesty's Government of The United Kingdom of Great Britain and (43) Date of publication of application: Northern Ireland Whitehall 02.12.81 Bulletin 81/48 London SW1 A 2HB (GB) (4jj) Publication ofthe grant of the patent: (7?) Inventor: Irvine, Stuart James Curzon 02.01 .85 Bulletin 85/01 14 Pixief ield, Cradley Malvern Worcestershire WR13 5ND (GB) Inventor: Mullin, John Brian (§) Designated Contracting States: The Hoo, Brockhill Road DE FR IT NL Malvern Worcestershire WR14 4DO (GB) (5B) References cited: @ Representative: Edwards, John Brian et al US-A-3 218 203 Procurement Executive Ministry of Defence US-A-3 664 866 Patents 1 A (4), Room 2014 Empress State US-A-3 725 135 Building Lillie Road London SW6 1TR (GB) J. Electrochem. Soc: Solid State Science & Technology, May 1981, pp. 1142-1144 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. -

Draft Report on Carcinogens Monograph on Antimony Trioxide
Draft Report on Carcinogens Monograph on Antimony Trioxide Peer-Review Draft November 29, 2017 Office of the Report on Carcinogens Division of the National Toxicology Program National Institute of Environmental Health Sciences U.S. Department of Health and Human Services This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally distributed by the National Toxicology Program. It does not represent and should not be construed to represent any NTP determination or policy. This Page Intentionally Left Blank Peer-Review Draft RoC Monograph on Antimony Trioxide 11/29/17 Foreword The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Report on Carcinogens (RoC) is prepared in response to Section 301 of the Public Health Service Act as amended. The RoC contains a list of identified substances (i) that either are known to be human carcinogens or are reasonably anticipated to be human carcinogens and (ii) to which a significant number of persons residing in the United States are exposed.