The List of Tungsten and Its Related Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film Wte2
Lawrence Berkeley National Laboratory Recent Work Title Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2. Permalink https://escholarship.org/uc/item/1642v6qf Journal Scientific reports, 8(1) ISSN 2045-2322 Authors Asaba, Tomoya Wang, Yongjie Li, Gang et al. Publication Date 2018-04-25 DOI 10.1038/s41598-018-24736-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificreports OPEN Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2 Received: 13 December 2017 Tomoya Asaba1, Yongjie Wang 2, Gang Li 1, Ziji Xiang1, Colin Tinsman1, Lu Chen1, Accepted: 5 April 2018 Shangnan Zhou1, Songrui Zhao2, David Laleyan2, Yi Li3, Zetian Mi2 & Lu Li 1 Published: xx xx xxxx In conventional superconductors an external magnetic feld generally suppresses superconductivity. This results from a simple thermodynamic competition of the superconducting and magnetic free energies. In this study, we report the unconventional features in the superconducting epitaxial thin flm tungsten telluride (WTe2). Measuring the electrical transport properties of Molecular Beam Epitaxy (MBE) grown WTe2 thin flms with a high precision rotation stage, we map the upper critical feld Hc2 at diferent temperatures T. We observe the superconducting transition temperature Tc is enhanced by in-plane magnetic felds. The upper critical feld Hc2 is observed to establish an unconventional non- monotonic dependence on temperature. We suggest that this unconventional feature is due to the lifting of inversion symmetry, which leads to the enhancement of Hc2 in Ising superconductors. Superconductivity generally competes with magnetic felds. Based on thermodynamics, an applied magnetic feld usually suppresses superconductivity by destroying the underlying electron pairing in the superconducting state1. -

Wte2): an Atomic Layered Semimetal
The Pennsylvania State University The Graduate School Department of Materials Science and Engineering TUNGSTEN DITELLURIDE (WTE2): AN ATOMIC LAYERED SEMIMETAL A Thesis in Materials Science and Engineering by Chia-Hui Lee 2015 Chia-Hui Lee Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science December 2015 ii The thesis of Chia-Hui Lee was reviewed and approved* by the following: Joshua A. Robinson Professor of Materials Science and Engineering Thesis Advisor Thomas E. Mallouk Head of the Chemistry Department Evan Pugh University Professor of Chemistry, Physics, Biochemistry and Molecular Biology Mauricio Terrones Professor of Physics, Chemistry and Materials Science & Engineering Suzanne Mohney Professor of Materials Science and Engineering and Electrical Engineering Chair, Intercollege Graduate Degree Program in Materials Science and Engineering *Signatures are on file in the Graduate School iii ABSTRACT Tungsten ditelluride (WTe2) is a transition metal dichalcogenide (TMD) with physical and electronic properties that make it attractive for a variety of electronic applications. Although WTe2 has been studied for decades, its structure and electronic properties have only recently been correctly described. We explored WTe2 synthesis via chemical vapor transport (CVT) method for bulk crystal, and chemical vapor deposition (CVD) routes for thin film material. We employed both experimental and theoretical techniques to investigate its structural, physical and electronic properties of WTe2, and verify that WTe2 has its minimum energy configuration in a distorted 1T structure (Td structure), which results in metallic-like behavior. Our findings confirmed the metallic nature of WTe2, introduce new information about the Raman modes of Td-WTe2, and demonstrate that Td- WTe2 is readily oxidized via environmental exposure. -

Appendix a of Final Environmental Impact Statement for a Geologic Repository for the Disposal of Spent Nuclear Fuel and High-Lev
Appendix A Inventory and Characteristics of Spent Nuclear Fuel, High-Level Radioactive Waste, and Other Materials Inventory and Characteristics of Spent Nuclear Fuel, High-Level Radioactive Waste, and Other Materials TABLE OF CONTENTS Section Page A. Inventory and Characteristics of Spent Nuclear Fuel, High-Level Radioactive Waste, and Other Materials ................................................................................................................................. A-1 A.1 Introduction .............................................................................................................................. A-1 A.1.1 Inventory Data Summary .................................................................................................... A-2 A.1.1.1 Sources ......................................................................................................................... A-2 A.1.1.2 Present Storage and Generation Status ........................................................................ A-4 A.1.1.3 Final Waste Form ......................................................................................................... A-6 A.1.1.4 Waste Characteristics ................................................................................................... A-6 A.1.1.4.1 Mass and Volume ................................................................................................. A-6 A.1.1.4.2 Radionuclide Inventories ...................................................................................... A-8 A.1.1.4.3 -

View PDF Version
Nanoscale Advances View Article Online REVIEW View Journal | View Issue In-plane anisotropic electronics based on low- symmetry 2D materials: progress and prospects Cite this: Nanoscale Adv., 2020, 2,109 Siwen Zhao, a Baojuan Dong,bc Huide Wang,a Hanwen Wang,bc Yupeng Zhang, a Zheng Vitto Han*bc and Han Zhang *a Low-symmetry layered materials such as black phosphorus (BP) have been revived recently due to their high intrinsic mobility and in-plane anisotropic properties, which can be used in anisotropic electronic and optoelectronic devices. Since the anisotropic properties have a close relationship with their anisotropic structural characters, especially for materials with low-symmetry, exploring new low- symmetry layered materials and investigating their anisotropic properties have inspired numerous research efforts. In this paper, we review the recent experimental progresses on low-symmetry layered Received 4th October 2019 materials and their corresponding anisotropic electrical transport, magneto-transport, optoelectronic, Accepted 30th October 2019 thermoelectric, ferroelectric, and piezoelectric properties. The boom of new low-symmetry layered DOI: 10.1039/c9na00623k materials with high anisotropy could open up considerable possibilities for next-generation anisotropic Creative Commons Attribution 3.0 Unported Licence. rsc.li/nanoscale-advances multifunctional electronic devices. 1. Introduction energy band structure and can be regarded as a process of lowering the dimensionality of the carrier transport. Therefore, Two dimensional (2D) layered materials with strong in-plane the electrical, optical, thermal, and phonon properties of these covalent bonds and weak out-of-plane van der Waals interac- anisotropic materials are diverse along the different in-plane tions span a very broad range of solids and exhibit extraordinary crystal directions. -
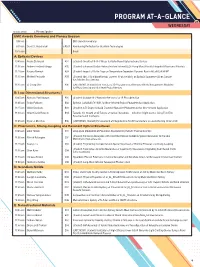
Program-At-A-Glance (PDF)
PROGRAM AT-A-GLANCE WEDNESDAY All times are EDT Plenary Speaker EMC Awards Ceremony and Plenary Session 9:00 am EMC Awards Ceremony 9:15 am David D. Awschalom PL01 Abandoning Perfection for Quantum Technologies 10:15 am Break A: Epitaxial Devices 10:45 am Rasha El-Jaroudi A01 (Student) Growth of B-III-V Alloys for GaAs-Based Optoelectronic Devices 11:00 am Andrew Frederick Briggs A02 (Student) Enhanced Double Heterostructure Infrared LEDs Using Monolithically Integrated Plasmonic Materials 11:15 am Nayana Remesh A03 (Student) Impact of Buffer Traps on Temperature-Dependent Dynamic Ron in AlGaN/GaN HEMT 11:30 am Michael Pedowitz A05 (Student) Mn+3 Rich Nanofiberous Layeredδ -phase MnO2 on Epitaxial Graphene-Silicon Carbide for Selective Gas Sensing 11:45 am Li-Chung Shih A06 (LATE NEWS, Student) Dual-Function ZTO Phototransistor Memory with Au Nanoparticles Mediated for Photo-Sensing and Multilevel Photo-Memory B: Low-Dimensional Structures I 10:45 am Nicholas Paul Morgan B01 (Student) Scalable III-V Nanowire Networks for IR Photodetection 11:00 am Rabin Pokharel B02 Epitaxial GaAsSbN (Te) NWs for Near-Infrared Region Photodetection Application 11:15 am Shisir Devakota B03 (Student) A Te Doped GaAsSb Ensemble Nanowire Photodetector for Near-Infrared Application 11:30 am Gilbert Daniel Nessim B04 Towards the Growth of 3D Forests of Carbon Nanotubes—Selective Height Control Using Thin-Film Reservoirs and Overlayers 11:45 am Dylan J. McIntyre B06 (LATE NEWS, Student) Enhancement of Integrated Cu-Ti-CNT Conductors via Joule-Heating Driven -

View PDF Version
Nanoscale View Article Online REVIEW View Journal | View Issue Synthesis of emerging 2D layered magnetic materials Cite this: Nanoscale, 2021, 13, 2157 Mauro Och,a Marie-Blandine Martin,b Bruno Dlubak, b Pierre Seneorb and Cecilia Mattevi *a van der Waals atomically thin magnetic materials have been recently discovered. They have attracted enormous attention as they present unique magnetic properties, holding potential to tailor spin-based device properties and enable next generation data storage and communication devices. To fully under- stand the magnetism in two-dimensions, the synthesis of 2D materials over large areas with precise thick- ness control has to be accomplished. Here, we review the recent advancements in the synthesis of these materials spanning from metal halides, transition metal dichalcogenides, metal phosphosulphides, to ternary metal tellurides. We initially discuss the emerging device concepts based on magnetic van der Waals materials including what has been achieved with graphene. We then review the state of the art of Creative Commons Attribution-NonCommercial 3.0 Unported Licence. the synthesis of these materials and we discuss the potential routes to achieve the synthesis of wafer- scale atomically thin magnetic materials. We discuss the synthetic achievements in relation to the struc- Received 3rd November 2020, tural characteristics of the materials and we scrutinise the physical properties of the precursors in relation Accepted 8th January 2021 to the synthesis conditions. We highlight the challenges related to the synthesis of 2D magnets and we DOI: 10.1039/d0nr07867k provide a perspective for possible advancement of available synthesis methods to respond to the need for rsc.li/nanoscale scalable production and high materials quality. -

Large-Area and High-Quality 2D Transition Metal Telluride
Large-area and high-quality 2D transition metal telluride Jiadong Zhou1†, Fucai Liu1†, Junhao Lin2,3,4*, Xiangwei Huang5†, Juan Xia6, Bowei Zhang1, Qingsheng Zeng1, Hong Wang1, Chao Zhu1, Lin Niu1, Xuewen Wang1, Wei Fu1, Peng Yu1, Tay- Rong Chang7, Chuang-Han Hsu8,9, Di Wu8,9, Horng-Tay Jeng7,10, Yizhong Huang1, Hsin Lin8,9, , Zexiang Shen1,6,11, Changli Yang5,12, Li Lu5,12, Kazu Suenaga4, Wu Zhou2, Sokrates T. Pantelides2,3, Guangtong Liu5* and Zheng Liu1, 13,14*. 1Centre for Programmable Materials, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore 2Materials Science and Technology Division, Oak Ridge National Lab, Oak Ridge Tennessee 37831, USA 3Department of Physics and Astronomy, Vanderbilt University, Nashville, TN 37235, USA 4National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba 305-8565, Japan 5Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China 6Division of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 7Department of Physics, National Tsing Hua University, Hsinchu 30013, Taiwan 8Centre for Advanced 2D Materials and Graphene Research Centre, National University of Singapore, Singapore 117546 9Department of Physics, National University of Singapore, Singapore 117542 10Institute of Physics, Academia Sinica, Taipei 11529, Taiwan 11Centre for Disruptive Photonic Technologies, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 12Collaborative Innovation Center of Quantum Matter, Beijing 100871, China 13Centre for Micro-/Nano-electronics (NOVITAS), School of Electrical & Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore 14CINTRA CNRS/NTU/THALES, UMI 3288, Research Techno Plaza, 50 Nanyang Drive, Border X Block, Level 6, Singapore 637553, Singapore † These authors contributed equally to this work. -

Surface and Interface Characterization of 2D Materials: Transition Metal Dichalcogenide and Black Phosphorous
SURFACE AND INTERFACE CHARACTERIZATION OF 2D MATERIALS: TRANSITION METAL DICHALCOGENIDE AND BLACK PHOSPHOROUS by Hui Zhu APPROVED BY SUPERVISORY COMMITTEE: ___________________________________________ Dr. Robert M. Wallace, Chair ___________________________________________ Dr. Christopher L. Hinkle ___________________________________________ Dr. Jiyoung Kim ___________________________________________ Dr. Kyeongjae Cho Copyright 2017 Hui Zhu All Rights Reserved Dedicated to my husband and my parents SURFACE AND INTERFACE CHARACTERIZATION OF 2D MATERIALS: TRANSITION METAL DICHALCOGENIDE AND BLACK PHOSPHOROUS by HUI ZHU, BS, MS DISSERTATION Presented to the Faculty of The University of Texas at Dallas in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN MATERIALS SCIENCE AND ENGINEERING THE UNIVERSITY OF TEXAS AT DALLAS December 2017 ACKNOWLEDGMENTS First of all, I’d like to thank my supervisor, Dr. Robert M. Wallace, for his continuous and tremendous support of my master’s and Ph.D. research, for his patience, motivation, and immense knowledge. His diligent working attitude and enthusiasm for science establish a great standard of learning for every researcher in our group, including me. An open, friendly, and positive working environment is created, and it is my pleasure to work and study there. I would also like to acknowledge my committee professors, Dr. Jiyoung Kim, Dr. Kyeongjae Cho, and Dr. Christopher L. Hinkle for their stimulating instruction and the supportive research environment they provided. Grateful appreciation is also delivered to my colleague Mr. Qingxiao Wang and his supervisor Dr. Moon J. Kim for their extensive STEM collaborations included in this work. My acknowledgment to all my colleagues working together on 2D materials at UTD, including postdoctoral researchers: Dr. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

A Kinetic Study of the Strontium Extraction by Metallothermic Reduction Using Submerged Sro Powders Injection ⁎ R
Available online at www.sciencedirect.com Materials Letters 62 (2008) 637–640 www.elsevier.com/locate/matlet A kinetic study of the strontium extraction by metallothermic reduction using submerged SrO powders injection ⁎ R. Muñiz a, , A. Flores a, J. Torres a, S. Luna a, N. Rodríguez b a CINVESTAV Unidad Saltillo, Saltillo-Monterrey highway Km. 13.5 P.O. Box 663, 25000, Saltillo, Coahuila, México b Instituto Tecnológico de Saltillo V. Carranza Blvd. 2500, Saltillo, Coahuila, México Received 3 May 2007; accepted 8 June 2007 Available online 16 June 2007 Abstract This work reports the results of laboratory experiments conduced to follow the kinetics of strontium recovery into the Al–Mg alloy by metallothermic reduction of SrO. The reagent was incorporated to molten alloy by the use of submerged powders injection technique. The variables analyzed were the injection time, the melt temperature and the initial magnesium content. Magnesium is added to the melt to increase the reactivity and reduce the surface tension of the molten aluminum. It was possible to increase the strontium content from 0 to 5 wt.% after 60 min of treatment. The results were fitted to a general kinetic equation, which allowed it to obtain the kinetic parameters, i.e. order of reaction and activation energy of the process. As the main mechanism of the strontium recovery process is of diffusive type, the global process rate increases as the temperature and initial amount of the magnesium increased. © 2007 Elsevier B.V. All rights reserved. Keywords: Strontium recovery; Submerged powder injection; Metallothermic reduction; Reaction kinetic 1. Introduction a vapour by vacuum distillation [2]. -

Synthesis Target Structures for Alkaline Earth Oxide Clusters
inorganics Article Synthesis Target Structures for Alkaline Earth Oxide Clusters Susanne G. E. T. Escher, Tomas Lazauskas ID , Martijn A. Zwijnenburg and Scott M. Woodley * ID Department of Chemistry, University College London, London WC1H 0AJ, UK; [email protected] (S.G.E.T.E.); [email protected] (T.L.); [email protected] (M.A.Z.) * Correspondence: [email protected] Received: 21 November 2017; Accepted: 7 February 2018; Published: 21 February 2018 Abstract: Knowing the possible structures of individual clusters in nanostructured materials is an important first step in their design. With previous structure prediction data for BaO nanoclusters as a basis, data mining techniques were used to investigate candidate structures for magnesium oxide, calcium oxide and strontium oxide clusters. The lowest-energy structures and analysis of some of their structural properties are presented here. Clusters that are predicted to be ideal targets for synthesis, based on being both the only thermally accessible minimum for their size, and a size that is thermally accessible with respect to neighbouring sizes, include global minima for: sizes n = 9, 15, 16, 18 and 24 for (MgO)n; sizes n = 8, 9, 12, 16, 18 and 24 for (CaO)n; the greatest number of sizes of (SrO)n clusters (n = 8, 9, 10, 12, 13, 15, 16, 18 and 24); and for (BaO)n sizes of n = 8, 10 and 16. Keywords: inorganic nanoclusters; global optimization; data mining; computational modelling; magnesium oxide; calcium oxide; strontium oxide; barium oxide 1. Introduction Structure determination of materials plays an important role in materials design because the properties of materials are inherently linked to their atomic and electronic structure. -

Exam 3 Review Course: Chem 177 Supplemental Instruction Instructor: Burnett Iowa State University Date: April 1St, 2010 1
Leader: Katie Exam 3 Review Course: Chem 177 Supplemental Instruction Instructor: Burnett Iowa State University Date: April 1st, 2010 1. From the enthalpies of reaction 퐻2 푔 + 퐹2 푔 → 2퐻퐹 푔 ∆퐻 = −537 푘퐽 퐶 푠 + 2퐹2 푔 → 퐶퐹4 푔 ∆퐻 = −680 푘퐽 2퐶 푠 + 2퐻2 푔 → 퐶2퐻4 푔 ∆퐻 = 52.3 푘퐽 Calculate ∆퐻 for the reaction of ethylene with 퐹2: 퐶2퐻4 푔 + 6퐹2 푔 → 2퐶퐹4 푔 + 4퐻퐹 푔 2. Calculate the standard enthalpy change for the following reaction using the values given in the table below. 2푆푂2 푔 + 푂2 푔 → 2푆푂3 푔 푆푂2 푔 -296.9 푆푂3 푔 -395.2 퐻2푂 푙 -285.8 3. Indicate whether each of the following statements is True or False. a) ______ Longer wavelengths of electromagnetic radiation correspond to greater frequencies. b) ______ Photon energies decrease with increasing wavelength. c) ______ The energy transmitted by electromagnetic radiation is quantized. 4. In the Bohr model of the atom ______. a) A single photon is absorbed when an electron moves from a low energy orbit to a higher energy orbit. b) Electrons freely roam throughout the volume of the atom. c) Electrons exist simultaneously in multiple orbits. d) Energy is absorbed when electrons fall to orbits close to the nucleus. e) Line spectra cannot be predicted for the hydrogen atom. 5. The light-sensitive substance in black-and-white photographic film is AgBr. Photons provide the energy necessary to transfer an electron from 퐵푟− 푡표 퐴푔+ to produce Ag and Br and darken the film. 2.00 x103 J/mol is the minimum amount of energy required for this process.