Wte2): an Atomic Layered Semimetal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UC Riverside UC Riverside Electronic Theses and Dissertations
UC Riverside UC Riverside Electronic Theses and Dissertations Title Designing New Structures of Magnetic Materials: Cases of Metal Borides and Metal Chalcogenides Permalink https://escholarship.org/uc/item/9ns4w70p Author Scheifers, Jan Phillip Publication Date 2020 License https://creativecommons.org/licenses/by-nc-sa/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Designing New Structures of Magnetic Materials: Cases of Metal Borides and Metal Chalcogenides A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry by Jan Phillip Scheifers March 2020 Dissertation Committee: Dr. Boniface B. T. Fokwa, Chairperson Dr. Ming Lee Tang Dr.Yadong Yin Copyright by Jan Phillip Scheifers 2020 The Dissertation of Jan Phillip Scheifers is approved: Committee Chairperson University of California, Riverside Für Papa iv Acknowledgments Some of the research presented in here has previously been published as: Jan P. Scheifers, Boniface P. T. Fokwa: Unprecedented Selective Substitution of Si by Cu in the New Ternary Silicide Ir4-xCuSi2. Manuscript submitted to Z. Kristallogr. Cryst. Mater. Ryland Forsythe, Jan P. Scheifers, Yuemei Zhang, Boniface Tsinde Polequin Fokwa: HT‐ NbOsB: Experimental and Theoretical Investigations of a New Boride Structure Type Containing Boron Chains and Isolated Boron Atoms. European Journal of Inorganic Chemistry 02/2018; 2018(28)., DOI:10.1002/ejic.201800235 Jan P. Scheifers, Michael Küpers, Rashid Touzani, Fabian C. Gladisch, Rainer Poettgen, Boniface P. T. Fokwa: 1D iron chains in the complex metal-rich boride Ti5-xFe1- yOs6+x+yB6 (x = 0.66(1), y = 0.27(1)) representing an unprecedented structure type based on unit cell twinning. -

NON-HAZARDOUS CHEMICALS May Be Disposed of Via Sanitary Sewer Or Solid Waste
NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste (+)-A-TOCOPHEROL ACID SUCCINATE (+,-)-VERAPAMIL, HYDROCHLORIDE 1-AMINOANTHRAQUINONE 1-AMINO-1-CYCLOHEXANECARBOXYLIC ACID 1-BROMOOCTADECANE 1-CARBOXYNAPHTHALENE 1-DECENE 1-HYDROXYANTHRAQUINONE 1-METHYL-4-PHENYL-1,2,5,6-TETRAHYDROPYRIDINE HYDROCHLORIDE 1-NONENE 1-TETRADECENE 1-THIO-B-D-GLUCOSE 1-TRIDECENE 1-UNDECENE 2-ACETAMIDO-1-AZIDO-1,2-DIDEOXY-B-D-GLYCOPYRANOSE 2-ACETAMIDOACRYLIC ACID 2-AMINO-4-CHLOROBENZOTHIAZOLE 2-AMINO-2-(HYDROXY METHYL)-1,3-PROPONEDIOL 2-AMINOBENZOTHIAZOLE 2-AMINOIMIDAZOLE 2-AMINO-5-METHYLBENZENESULFONIC ACID 2-AMINOPURINE 2-ANILINOETHANOL 2-BUTENE-1,4-DIOL 2-CHLOROBENZYLALCOHOL 2-DEOXYCYTIDINE 5-MONOPHOSPHATE 2-DEOXY-D-GLUCOSE 2-DEOXY-D-RIBOSE 2'-DEOXYURIDINE 2'-DEOXYURIDINE 5'-MONOPHOSPHATE 2-HYDROETHYL ACETATE 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID 2-METHYLFLUORENE 2-METHYL-2-THIOPSEUDOUREA SULFATE 2-MORPHOLINOETHANESULFONIC ACID 2-NAPHTHOIC ACID 2-OXYGLUTARIC ACID 2-PHENYLPROPIONIC ACID 2-PYRIDINEALDOXIME METHIODIDE 2-STEP CHEMISTRY STEP 1 PART D 2-STEP CHEMISTRY STEP 2 PART A 2-THIOLHISTIDINE 2-THIOPHENECARBOXYLIC ACID 2-THIOPHENECARBOXYLIC HYDRAZIDE 3-ACETYLINDOLE 3-AMINO-1,2,4-TRIAZINE 3-AMINO-L-TYROSINE DIHYDROCHLORIDE MONOHYDRATE 3-CARBETHOXY-2-PIPERIDONE 3-CHLOROCYCLOBUTANONE SOLUTION 3-CHLORO-2-NITROBENZOIC ACID 3-(DIETHYLAMINO)-7-[[P-(DIMETHYLAMINO)PHENYL]AZO]-5-PHENAZINIUM CHLORIDE 3-HYDROXYTROSINE 1 9/26/2005 NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste 3-HYDROXYTYRAMINE HYDROCHLORIDE 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE -

Impact of Back Surface Field (BSF) Layers in Cadmium Telluride (Cdte) Solar Cells from Numerical Analysis
Impact of Back Surface Field (BSF) Layers in Cadmium Telluride (CdTe) Solar Cells from Numerical Analysis C. Doroody1,a), K.S. Rahman2,b), H.N. Rosly1, M.N. Harif1, Y. Yusoff2, S. Fazlili1, M.A. Matin3, S.K. Tiong2 and N. Amin2,c) 1College of Engineering, Universiti Tenaga Nasional (@The National Energy University), Jalan IKRAM- UNITEN, 43000 Kajang, Selangor, Malaysia 2Institute of Sustainable Energy, Universiti Tenaga Nasional (@The National Energy University), Jalan IKRAM-UNITEN, 43000 Kajang, Selangor, Malaysia 3Renewable Energy Laboratory, Chittagong University of Engineering and Technology, Chittagong 4349, Bangladesh a)Corresponding author: [email protected] b)[email protected] c)[email protected] Abstract. Numerical simulation has been executed using Solar Cell Capacitance Simulator (SCAPS-1D) to study the possibility of favourable efficiency and stable CdS/CdTe cell in various cell configurations. A basic structure of CdS/CdTe cell is studied in this work with 4 m CdTe absorber layer and 100 nm tin oxide (SnO2) as front contact, 25 nm cadmium sulfide (CdS) as buffer layer, zinc telluride (ZnTe) is used as back surface field (BSF) material compared with ZnTe:Cu, Cu2Te and MoTe2 in order to reduce the minority carrier recombination at back surface field (BSF). The cell structure of glass/SnO2/CdS/CdTe/MoTe2 has shown the highest conversion 2 efficiency of 17.04% (Voc=0.91V, Jsc=24.79 mA/cm , FF=75.41). These calculations have verified that SnO2 as buffer layer and MoTe2 as back contacts are suitable for an efficient CdS/CdTe cell. Also, it is found that a few nanometers (about 40 nm) of back surface layer is enough to achieve high conversion efficiency. -

Research of the Boundary of the Section of a Photo Receiver Based on Mos Pcdte / Cdo Structures
The American Journal of Applied Sciences IMPACT FACTOR – (ISSN 2689-0992) 2020: 5. 276 Published: October 31, 2020 | Pages: 97-105 Doi: https://doi.org/10.37547/tajas/Volume02Issue10-14 OCLC - 1121105553 Research Of The Boundary Of The Section Of A Photo Receiver Based On Mos pCdTe / CdO Structures Sh.B.Utamuradova Research Institute Of Semiconductor Physics And Microelectronics At The National University Of Uzbekistan Named After Mirzo Ulugbek, Tashkent, Uzbekistan S.A.Muzafarova Journal Website: Research Institute Of Semiconductor Physics And Microelectronics At The National University http://usajournalshub.c Of Uzbekistan Named After Mirzo Ulugbek, Tashkent, Uzbekistan om/index,php/tajas Copyright: Original content from this work may be used under the terms of the creative commons attributes 4.0 licence. ABSTRACT In this work, we investigated an intermediate layer in the structure of a photosensitive MOS structure nCdO / pCdTe . X-ray phase analysis shows that the intermediate layer between Mo and pCdTe is rather complex in composition. It contains dichalcogenides - three oxide MoO3 and a thin layer of a composite material with the composition of ditelluride MoTe2 . According to X-ray diffraction measurements, the total thickness of the intermediate layer is no more than ~ 200Ǻ. It was shown that in the nCdO / pCdTe structure the base material CdTe mainly consists of a homogeneous cubic modification layer. KEYWORDS Intermediate layer, photosensitivity , gas transport, interfaces , dichalcogenides , X-ray diffraction analysis, structure. -

Synthesis and Characterization of Mote2 Thin Films for Photoelectro-Chemical Cell Applications
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universiti Teknikal Malaysia Melaka (UTeM) Repository Synthesis and Characterization of MoTe2 Thin Films for Photoelectro-chemical Cell Applications Lim Mei Ying*, Nor Hamizah Bt. Mazlan and T. Joseph Sahaya Anand Department of Engineering Materials, Faculty of Manufacturing Engineering, University Technical Malaysia Melaka, Durian Tunggal Melaka 76100, Malaysia. Abstract - Thin films of transition metal chalcogenides, molybdenum ditelluride (MoTe2) have been electrodeposited cathodically on indium tin oxide-coated conducting glass substrates from ammonaical solution of H2MoO4 and TeO2. These MoTe2 thin films are useful as photovoltaic cell and photoelectro-chemical (PEC) solar cell. The electrode potential was varied while the bath temperature was maintained at 40±1 ºC and deposition time of 30 minutes. X-ray diffraction analysis showed the presence of highly textured MoTe2 films with polycrystalline nature. Compositional analysis by EDX gives their stoichiometric relationships. Scanning electron microscope (SEM) was used to study surface morphology and shows that the films are smooth, uniform and useful for device fabrication. The optical absorption spectra showed that the material has an indirect band- gap value of 1.91-2.04 eV with different electrode potential. Besides, the film exhibited p-type semiconductor behavior. Keywords: Molybdenum ditelluride; Thin films; Electrodepositon; Solar cell 1. INTRODUCTION using X-ray diffraction analysis, optical studies and SEM Recently there has been a growing interest in layered /EDX studies. semiconducting compounds consisting of group VI transition metal dichalcogenides MX2 (M = Mo, W, Cd, Ni etc. and X = 2. EXPERIMENTAL S, Se, Te) [1-3]. -

Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film Wte2
Lawrence Berkeley National Laboratory Recent Work Title Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2. Permalink https://escholarship.org/uc/item/1642v6qf Journal Scientific reports, 8(1) ISSN 2045-2322 Authors Asaba, Tomoya Wang, Yongjie Li, Gang et al. Publication Date 2018-04-25 DOI 10.1038/s41598-018-24736-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificreports OPEN Magnetic Field Enhanced Superconductivity in Epitaxial Thin Film WTe2 Received: 13 December 2017 Tomoya Asaba1, Yongjie Wang 2, Gang Li 1, Ziji Xiang1, Colin Tinsman1, Lu Chen1, Accepted: 5 April 2018 Shangnan Zhou1, Songrui Zhao2, David Laleyan2, Yi Li3, Zetian Mi2 & Lu Li 1 Published: xx xx xxxx In conventional superconductors an external magnetic feld generally suppresses superconductivity. This results from a simple thermodynamic competition of the superconducting and magnetic free energies. In this study, we report the unconventional features in the superconducting epitaxial thin flm tungsten telluride (WTe2). Measuring the electrical transport properties of Molecular Beam Epitaxy (MBE) grown WTe2 thin flms with a high precision rotation stage, we map the upper critical feld Hc2 at diferent temperatures T. We observe the superconducting transition temperature Tc is enhanced by in-plane magnetic felds. The upper critical feld Hc2 is observed to establish an unconventional non- monotonic dependence on temperature. We suggest that this unconventional feature is due to the lifting of inversion symmetry, which leads to the enhancement of Hc2 in Ising superconductors. Superconductivity generally competes with magnetic felds. Based on thermodynamics, an applied magnetic feld usually suppresses superconductivity by destroying the underlying electron pairing in the superconducting state1. -

View PDF Version
Nanoscale Advances View Article Online REVIEW View Journal | View Issue In-plane anisotropic electronics based on low- symmetry 2D materials: progress and prospects Cite this: Nanoscale Adv., 2020, 2,109 Siwen Zhao, a Baojuan Dong,bc Huide Wang,a Hanwen Wang,bc Yupeng Zhang, a Zheng Vitto Han*bc and Han Zhang *a Low-symmetry layered materials such as black phosphorus (BP) have been revived recently due to their high intrinsic mobility and in-plane anisotropic properties, which can be used in anisotropic electronic and optoelectronic devices. Since the anisotropic properties have a close relationship with their anisotropic structural characters, especially for materials with low-symmetry, exploring new low- symmetry layered materials and investigating their anisotropic properties have inspired numerous research efforts. In this paper, we review the recent experimental progresses on low-symmetry layered Received 4th October 2019 materials and their corresponding anisotropic electrical transport, magneto-transport, optoelectronic, Accepted 30th October 2019 thermoelectric, ferroelectric, and piezoelectric properties. The boom of new low-symmetry layered DOI: 10.1039/c9na00623k materials with high anisotropy could open up considerable possibilities for next-generation anisotropic Creative Commons Attribution 3.0 Unported Licence. rsc.li/nanoscale-advances multifunctional electronic devices. 1. Introduction energy band structure and can be regarded as a process of lowering the dimensionality of the carrier transport. Therefore, Two dimensional (2D) layered materials with strong in-plane the electrical, optical, thermal, and phonon properties of these covalent bonds and weak out-of-plane van der Waals interac- anisotropic materials are diverse along the different in-plane tions span a very broad range of solids and exhibit extraordinary crystal directions. -
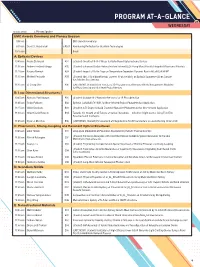
Program-At-A-Glance (PDF)
PROGRAM AT-A-GLANCE WEDNESDAY All times are EDT Plenary Speaker EMC Awards Ceremony and Plenary Session 9:00 am EMC Awards Ceremony 9:15 am David D. Awschalom PL01 Abandoning Perfection for Quantum Technologies 10:15 am Break A: Epitaxial Devices 10:45 am Rasha El-Jaroudi A01 (Student) Growth of B-III-V Alloys for GaAs-Based Optoelectronic Devices 11:00 am Andrew Frederick Briggs A02 (Student) Enhanced Double Heterostructure Infrared LEDs Using Monolithically Integrated Plasmonic Materials 11:15 am Nayana Remesh A03 (Student) Impact of Buffer Traps on Temperature-Dependent Dynamic Ron in AlGaN/GaN HEMT 11:30 am Michael Pedowitz A05 (Student) Mn+3 Rich Nanofiberous Layeredδ -phase MnO2 on Epitaxial Graphene-Silicon Carbide for Selective Gas Sensing 11:45 am Li-Chung Shih A06 (LATE NEWS, Student) Dual-Function ZTO Phototransistor Memory with Au Nanoparticles Mediated for Photo-Sensing and Multilevel Photo-Memory B: Low-Dimensional Structures I 10:45 am Nicholas Paul Morgan B01 (Student) Scalable III-V Nanowire Networks for IR Photodetection 11:00 am Rabin Pokharel B02 Epitaxial GaAsSbN (Te) NWs for Near-Infrared Region Photodetection Application 11:15 am Shisir Devakota B03 (Student) A Te Doped GaAsSb Ensemble Nanowire Photodetector for Near-Infrared Application 11:30 am Gilbert Daniel Nessim B04 Towards the Growth of 3D Forests of Carbon Nanotubes—Selective Height Control Using Thin-Film Reservoirs and Overlayers 11:45 am Dylan J. McIntyre B06 (LATE NEWS, Student) Enhancement of Integrated Cu-Ti-CNT Conductors via Joule-Heating Driven -

View PDF Version
Nanoscale View Article Online REVIEW View Journal | View Issue Synthesis of emerging 2D layered magnetic materials Cite this: Nanoscale, 2021, 13, 2157 Mauro Och,a Marie-Blandine Martin,b Bruno Dlubak, b Pierre Seneorb and Cecilia Mattevi *a van der Waals atomically thin magnetic materials have been recently discovered. They have attracted enormous attention as they present unique magnetic properties, holding potential to tailor spin-based device properties and enable next generation data storage and communication devices. To fully under- stand the magnetism in two-dimensions, the synthesis of 2D materials over large areas with precise thick- ness control has to be accomplished. Here, we review the recent advancements in the synthesis of these materials spanning from metal halides, transition metal dichalcogenides, metal phosphosulphides, to ternary metal tellurides. We initially discuss the emerging device concepts based on magnetic van der Waals materials including what has been achieved with graphene. We then review the state of the art of Creative Commons Attribution-NonCommercial 3.0 Unported Licence. the synthesis of these materials and we discuss the potential routes to achieve the synthesis of wafer- scale atomically thin magnetic materials. We discuss the synthetic achievements in relation to the struc- Received 3rd November 2020, tural characteristics of the materials and we scrutinise the physical properties of the precursors in relation Accepted 8th January 2021 to the synthesis conditions. We highlight the challenges related to the synthesis of 2D magnets and we DOI: 10.1039/d0nr07867k provide a perspective for possible advancement of available synthesis methods to respond to the need for rsc.li/nanoscale scalable production and high materials quality. -

Large-Area and High-Quality 2D Transition Metal Telluride
Large-area and high-quality 2D transition metal telluride Jiadong Zhou1†, Fucai Liu1†, Junhao Lin2,3,4*, Xiangwei Huang5†, Juan Xia6, Bowei Zhang1, Qingsheng Zeng1, Hong Wang1, Chao Zhu1, Lin Niu1, Xuewen Wang1, Wei Fu1, Peng Yu1, Tay- Rong Chang7, Chuang-Han Hsu8,9, Di Wu8,9, Horng-Tay Jeng7,10, Yizhong Huang1, Hsin Lin8,9, , Zexiang Shen1,6,11, Changli Yang5,12, Li Lu5,12, Kazu Suenaga4, Wu Zhou2, Sokrates T. Pantelides2,3, Guangtong Liu5* and Zheng Liu1, 13,14*. 1Centre for Programmable Materials, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore 2Materials Science and Technology Division, Oak Ridge National Lab, Oak Ridge Tennessee 37831, USA 3Department of Physics and Astronomy, Vanderbilt University, Nashville, TN 37235, USA 4National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba 305-8565, Japan 5Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China 6Division of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 7Department of Physics, National Tsing Hua University, Hsinchu 30013, Taiwan 8Centre for Advanced 2D Materials and Graphene Research Centre, National University of Singapore, Singapore 117546 9Department of Physics, National University of Singapore, Singapore 117542 10Institute of Physics, Academia Sinica, Taipei 11529, Taiwan 11Centre for Disruptive Photonic Technologies, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore 12Collaborative Innovation Center of Quantum Matter, Beijing 100871, China 13Centre for Micro-/Nano-electronics (NOVITAS), School of Electrical & Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore 14CINTRA CNRS/NTU/THALES, UMI 3288, Research Techno Plaza, 50 Nanyang Drive, Border X Block, Level 6, Singapore 637553, Singapore † These authors contributed equally to this work. -

WO 2014/062135 Al 24 April 2014 (24.04.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/062135 Al 24 April 2014 (24.04.2014) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, B32B 5/16 (2006.01) H01L 23/29 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, B32B 27/04 (2006.01) H01L 51/52 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, B32B 19/02 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/SG201 3/000448 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 18 October 2013 (18.10.201 3) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 61/715,420 18 October 2012 (18. 10.2012) US UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (71) Applicant: TERA-BARRIER FILMS PTE LTD EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, [SG/SG]; 3, Research Link, Singapore 117602 (SG). -

United States Patent 0 "Ice Patented May 28, 1968
3,385,667 United States Patent 0 "ice Patented May 28, 1968 1 2 3,385,667 given the designation ASTM 15-658. Calculated lattice MULYBDENUM DITELLURIDE AND HIGH parameters were (123.5182 A., and 0:13.9736 A., the TEMPERATURE, HIGH - PRESSURE SYN calculated density was 7.784 g./cc. THESES METHOD OF PREPARING SAME L. H. Brixner, Journal Inorganic Nuclear Chemistry, ieyer Shea Siiverman, Norristown, Pa., assigncr to Penn vol. 24, pp. 257—-65, (1962), prepared MoTe2 in single salt Chemicals Corporation, Philadelphia, Pa, a cor crystal form by means of transport reactions using bro poration of Pennsylvania mine as the transport agent. Crystallographic data was No Drawing. Filed Feb. 11, 1966, Ser. No. 526,702 taken on the basis of single crystal patterns using K a 4 Claims. (Cl. 23-204) radiation of Cu()t=1.5418 A). Lattice parameters were 10 (1:3.517 A. and 0:13.949 A., which constants were in ABSTRACT OF THE DISCLOSURE good agreement with those of Puotinen and Newnham, and Knop and MacDonald, supra. The MoTe2 exhibited A new form of molybdenum ditelluride having a rhom good semi-conducting properties having an electrical bohedral crystal structure is prepared by subjecting a mix resistivity measured at 25° C. and at —-196° C. of 8.5 ture of molybdenum and tellurium to a temperature of at and 1.34>< l03 ohm-cm, respectively. least about 1700° C. and a pressure of at least about 15 It is also reported by the US. Department of Com kilobars. merce, Ot?ce of Technical Service, Bulletin AD 265,121 (1961) that small single crystals of MoTe2 were obtained This invention relates to a new form of molybdenum by chemical transport reaction with bromine as the trans ditelluride and to a method of preparing molybdenum 20 port agent.