The Si Metric System of Units and Seg Tentative Metric Standard
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Representation of Derived Units in Unitsml (Revised December 8, 2006)
Representation of Derived Units in UnitsML (revised December 8, 2006) Peter J. Linstrom∗ December 8, 2006 ∗phone: (301) 975-5422,DRAFT e-mail: [email protected] 1 INTRODUCTION 12/8/06 Contents 1 Introduction 1 2 Why this convention is needed 2 3 Information needed to define a unit 3 4 Proposed XML encoding 4 5 Important conventions 7 6 Potential problems 8 7 Possible alternatives 10 A Multiplicative prefixes 12 B SI units and units acceptable for use with the SI 14 C non-SI Units 19 1 Introduction This document describes a proposed convention for defining derived units in terms of their base units. This convention is intended for use in the UnitsML markup language to allow a precise definition of a wide range of units. The goal of this convention is to improve interoperability among applications and databases which use derived units based on commonly encountered base units. It is understoodDRAFT that not all units can be represented using this convention. It is, however, Representation of Derived Units in UnitsML (revised December 8, 2006) Page 1 2 WHY THIS CONVENTION IS NEEDED 12/8/06 anticipated that a wide range of scientific and engineering units of measure can be represented with this convention. The convention consists of representing the unit in terms of multiplicative combinations of base units. For example the unit centimeter per second squared would be represented in terms of the following: 1. The unit meter with the prefix centi raised to the power 1. 2. The unit second raised to the power −2. -

Astm Metric Practice Guide
National Bureau of Standards Library, E-01 Admrn. Bldg. A111DO cmfl4S - m 2 0 1967 METRIC PRACTICE GUIDE NATL INST OF STANDARDS & TECH R.I.C. All100991845 /NBS handbook QC1 .U51 V102;1967 C.1 NBS-PUB-C 1934 U. S. DEPARTMENT OF COMMERCE NATIONAL OUREAU OF STANDARDS HANDBOOK 102 THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation’s scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific com¬ munity, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics — Electricity—Metrology — Mechanics — Heat — Atomic Physics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics2—Radio Standards Laboratory,2 which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Reference Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of materials. Beyond its direct interest to the Nation’s scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

The Beautiful Cubit System I Douglas 2019 the Beautiful Cubit System
The Beautiful Cubit System I Douglas 2019 The Beautiful Cubit System Ian Douglas, B.Sc [email protected] 30 June 2019 Version 1.0.0 DOI: https://doi.org/10.5281/zenodo.3263864 This work is licensed under the Creative Commons Attribution 4.0 International License. Abstract An analysis of the Egyptian Royal cubit, presenting some research and opinions flowing from that research, into what I believe was the original cubit, and how it was corrupted. I show various close arithmetic approximations and multiple ways of getting the divisions of the cubit, as well as some related measures. The cubit also encapsulates the basic components for the metric system. Keywords: Egyptology, metrology, royal cubit, cubit, metre, foot, metric system Contents 1. Introduction 2. Overview of current understanding 3. An alternative origin 4. Different ways of approximating the royal cubit 5. Different ways of getting the cubit divisions 6. Geometry, the Royal Cubit and the metric system 7. Bibliography 1. Introduction The cubit is a well-know ancient measure of length, used around various places in the Middle East and Mediterranean region in the distant past. 1 The Beautiful Cubit System I Douglas 2019 It is allegedly based on the length of a human (male) fore-arm. It is typically measured from the back of the elbow to some point between the wrist and the end of the outstretched middle finger, or in some variants, a point beyond that. The problem with this approach is that everyone’s arm is a different length. If the heights of the dynastic Egyptians is taken as representative, then their arms would have been too short to justify the accepted lengths. -

Electric Units and Standards
DEPARTMENT OF COMMERCE OF THE Bureau of Standards S. W. STRATTON, Director No. 60 ELECTRIC UNITS AND STANDARDS list Edition 1 Issued September 25. 1916 WASHINGTON GOVERNMENT PRINTING OFFICE DEPARTMENT OF COMMERCE Circular OF THE Bureau of Standards S. W. STRATTON, Director No. 60 ELECTRIC UNITS AND STANDARDS [1st Edition] Issued September 25, 1916 WASHINGTON GOVERNMENT PRINTING OFFICE 1916 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 15 CENTS PER COPY A complete list of the Bureau’s publications may be obtained free of charge on application to the Bureau of Standards, Washington, D. C. ELECTRIC UNITS AND STANDARDS CONTENTS Page I. The systems of units 4 1. Units and standards in general 4 2. The electrostatic and electromagnetic systems 8 () The numerically different systems of units 12 () Gaussian systems, and ratios of the units 13 “ ’ (c) Practical ’ electromagnetic units 15 3. The international electric units 16 II. Evolution of present system of concrete standards 18 1. Early electric standards 18 2. Basis of present laws 22 3. Progress since 1893 24 III. Units and standards of the principal electric quantities 29 1. Resistance 29 () International ohm 29 Definition 29 Mercury standards 30 Secondary standards 31 () Resistance standards in practice 31 (c) Absolute ohm 32 2. Current 33 (a) International ampere 33 Definition 34 The silver voltameter 35 ( b ) Resistance standards used in current measurement 36 (c) Absolute ampere 37 3. Electromotive force 38 (a) International volt 38 Definition 39 Weston normal cell 39 (b) Portable Weston cells 41 (c) Absolute and semiabsolute volt 42 4. -

What Is a Rain Barrel?
What is a rain barrel? A rain barrel is a container used to collect and store rainwater from your roof that would otherwise be lost to runoff and diverted out onto your property or to a storm drain and eventually to local streams or rivers. Rain barrels are also an economical way to store rain water to be used as a secondary water supply for indoor plants, flower gardens, lawns, fill the bird bath, and washing cars and windows. Rain barrels are usually about 40-60 gallons and can be purchased or made relatively easily. The parts are available at any hardware store. *Stored water is not used for drinking or bathing* Why use rain barrels? Every time it rains, unabsorbed water rushes to storm drains and directly into our local waterways. Often times this runoff carries with it pollutants it has picked up along the way depositing in them into local waterways. Any rainwater in an urban or suburban area that does not evaporate or infiltrate into the ground is considered stormwater. Infiltration is when water on the ground surface soaks into the soil. Impervious surfaces like roofs, asphalt, and concrete do not allow Rain water from your roof and driveway travels to the street and into storm drains for the infiltration to occur. eventually draining into our creeks, lakes, and rivers. Infiltration of water on pervious surfaces is important because it reduces the amount runoff and the possibility of erosion and pollutants leaving a site and entering a waterway. What can rain barrels do for you? Healthier plants. -

Math Assessment 5.0A
Math Assessment 5.0A . Name:_______________________________Date: 1. Solve. 2. Solve. 3 x (6 +1) = [8 x (10 - 3)] * 12= a. iq a. 68 b. 21 b. 80 c. 10 c. 152 d. 16 d. 02 3. Solve. H. Add parentheses to make {[5 * (2 x 3)] x (3 x 6)}= the statement true. 8 x 10 + 5 = 120 a. 378 b. 108 a (8) x 10 * 5 c. 201 b. (8x0) *5 d. 388 c. 8 x (10 + 5) d. The statement is already true. 5. Compare 36 - (3 + 6) and 6. How do parentheses in an 36 - 3 ♦ 6. equation affect the answer? viam Assessmem 5.0A .2 N am e-_____________________ ___________ Date: 1. Choose the words that 2. Choose the numerical match the numerical expression that matches expression. (3 +13) x 2 the words. Add 6 and 6. then divide b y 2. a. Add three and thirteen multiplied by two. a. 6 + 6 * 2 b. Add three and thirteen, b. (6 *6) *2 then multiply by two. c. 2 * 6 + 6 c. Add three and thirteen, d. 2 * (6 + 6) then add two. 3. Choose the numerical H. Choose the words that expression that matches match the numerical the words. Multiply the sum expression. IHH * 12 o f 3 and 2 by the sum o f 5 and i a. 12 divided by IHH a. 3 x 2 + 5 x 1 b. Divide IHH by 12 b. 3 + 2 + 5 +1 c. IHH multiplied by 12 c. (3 + 2) x (5 +1) d. Divide 12 by IHH d. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

U.S.-Canada Cross- Border Petroleum Trade
U.S.-Canada Cross- Border Petroleum Trade: An Assessment of Energy Security and Economic Benefits March 2021 Submitted to: American Petroleum Institute 200 Massachusetts Ave NW Suite 1100, Washington, DC 20001 Submitted by: Kevin DeCorla-Souza ICF Resources L.L.C. 9300 Lee Hwy Fairfax, VA 22031 U.S.-Canada Cross-Border Petroleum Trade: An Assessment of Energy Security and Economic Benefits This report was commissioned by the American Petroleum Institute (API) 2 U.S.-Canada Cross-Border Petroleum Trade: An Assessment of Energy Security and Economic Benefits Table of Contents I. Executive Summary ...................................................................................................... 4 II. Introduction ................................................................................................................... 6 III. Overview of U.S.-Canada Petroleum Trade ................................................................. 7 U.S.-Canada Petroleum Trade Volumes Have Surged ........................................................... 7 Petroleum Is a Major Component of Total U.S.-Canada Bilateral Trade ................................. 8 IV. North American Oil Production and Refining Markets Integration ...........................10 U.S.-Canada Oil Trade Reduces North American Dependence on Overseas Crude Oil Imports ..................................................................................................................................10 Cross-Border Pipelines Facilitate U.S.-Canada Oil Market Integration...................................14 -

Units of Weight and Measure : Definitions and Tables of Equivalents
Units of Weight and Measure (United States Customary and Metric) Definitions and Tables of Equivalents United States Department of Commerce National Bureau of Standards Miscellaneous Publication 233 THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the fisting of divisions and sections on the inside of the back cover. Publications The results of the Bureau's work take the form of either actual equipment and devices -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
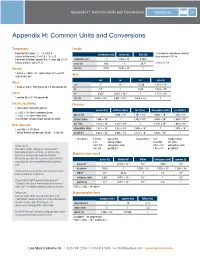
Appendix H: Common Units and Conversions Appendices 215
Appendix H: Common Units and Conversions Appendices 215 Appendix H: Common Units and Conversions Temperature Length Fahrenheit to Celsius: °C = (°F-32)/1.8 1 micrometer (sometimes referred centimeter (cm) meter (m) inch (in) Celsius to Fahrenheit: °F = (1.8 × °C) + 32 to as micron) = 10-6 m Fahrenheit to Kelvin: convert °F to °C, then add 273.15 centimeter (cm) 1 1.000 × 10–2 0.3937 -3 Celsius to Kelvin: add 273.15 meter (m) 100 1 39.37 1 mil = 10 in Volume inch (in) 2.540 2.540 × 10–2 1 –3 3 1 liter (l) = 1.000 × 10 cubic meters (m ) = 61.02 Area cubic inches (in3) cm2 m2 in2 circ mil Mass cm2 1 10–4 0.1550 1.974 × 105 1 kilogram (kg) = 1000 grams (g) = 2.205 pounds (lb) m2 104 1 1550 1.974 × 109 Force in2 6.452 6.452 × 10–4 1 1.273 × 106 1 newton (N) = 0.2248 pounds (lb) circ mil 5.067 × 10–6 5.067 × 10–10 7.854 × 10–7 1 Electric resistivity Pressure 1 micro-ohm-centimeter (µΩ·cm) pascal (Pa) millibar (mbar) torr (Torr) atmosphere (atm) psi (lbf/in2) = 1.000 × 10–6 ohm-centimeter (Ω·cm) –2 –3 –6 –4 = 1.000 × 10–8 ohm-meter (Ω·m) pascal (Pa) 1 1.000 × 10 7.501 × 10 9.868 × 10 1.450 × 10 = 6.015 ohm-circular mil per foot (Ω·circ mil/ft) millibar (mbar) 1.000 × 102 1 7.502 × 10–1 9.868 × 10–4 1.450 × 10–2 2 0 –3 –2 Heat flow rate torr (Torr) 1.333 × 10 1.333 × 10 1 1.316 × 10 1.934 × 10 5 3 2 1 1 watt (W) = 3.413 Btu/h atmosphere (atm) 1.013 × 10 1.013 × 10 7.600 × 10 1 1.470 × 10 1 British thermal unit per hour (Btu/h) = 0.2930 W psi (lbf/in2) 6.897 × 103 6.895 × 101 5.172 × 101 6.850 × 10–2 1 1 torr (Torr) = 133.332 pascal (Pa) 1 pascal (Pa) = 0.01 millibar (mbar) 1.33 millibar (mbar) 0.007501 torr (Torr) –6 A Note on SI 0.001316 atmosphere (atm) 9.87 × 10 atmosphere (atm) 2 –4 2 The values in this catalog are expressed in 0.01934 psi (lbf/in ) 1.45 × 10 psi (lbf/in ) International System of Units, or SI (from the Magnetic induction B French Le Système International d’Unités).