Protective Role of Salt in Catalysis and Maintaining Structure of Halophilic Proteins Against Denaturation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
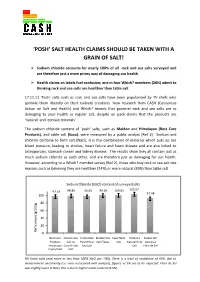
Latest Press Release from Consensus Action on Salt and Health
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

1.5. Raman Spectroscopy
Open Research Online The Open University’s repository of research publications and other research outputs Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Thesis How to cite: Rolfe, Samantha (2017). Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration. PhD thesis The Open University. For guidance on citations see FAQs. c 2016 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.0000c66a Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Samantha Melanie Rolfe MPhys (Hons), University of Leicester, 2010 September 2016 The Open University School of Physical Sciences A THESIS SUBMITTED TO THE OPEN UNIVERSITY IN THE SUBJECT OF PLANETARY SCIENCES FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Acknowledgements To my parents, Mark and Melanie, and my brother, Alex, who have always supported and encouraged me to follow my dreams and overcome all and any obstacles to achieve them. To my grandparents, Anthony and Margery Wilson, Aubrey and Val Rolfe and Marjorie and Russell Whitmore, this work is dedicated to you. To Chris, my rock, without you I absolutely would not have been able to get through this process. -

Insights on Cadmium Removal by Bioremediation: the Case of Haloarchaea
Review Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea Mónica Vera-Bernal 1 and Rosa María Martínez-Espinosa 1,2,* 1 Biochemistry and Molecular Biology Division, Agrochemistry and Biochemistry Department, Faculty of Sciences, University of Alicante, Ap. 99, E-03080 Alicante, Spain; [email protected] 2 Multidisciplinary Institute for Environmental Studies “Ramón Margalef”, University of Alicante, Ap. 99, E-03080 Alicante, Spain * Correspondence: [email protected]; Tel.: +34-965903400 (ext. 1258; 8841) Abstract: Although heavy metals are naturally found in the environment as components of the earth’s crust, environmental pollution by these toxic elements has increased since the industrial revolution. Some of them can be considered essential, since they play regulatory roles in different biological processes; but the role of other heavy metals in living tissues is not clear, and once ingested they can accumulate in the organism for long periods of time causing adverse health effects. To mitigate this problem, different methods have been used to remove heavy metals from water and soil, such as chelation-based processes. However, techniques like bioremediation are leaving these conventional methodologies in the background for being more effective and eco-friendlier. Recently, different research lines have been promoted, in which several organisms have been used for bioremediation approaches. Within this context, the extremophilic microorganisms represent one of the best tools for the treatment of contaminated sites due to the biochemical and molecular properties they show. Furthermore, since it is estimated that 5% of industrial effluents are saline and hypersaline, halophilic microorganisms have been suggested as good candidates for bioremediation Citation: Vera-Bernal, M.; and treatment of this kind of samples. -

Life at Low Water Activity
Published online 12 July 2004 Life at low water activity W. D. Grant Department of Infection, Immunity and Inflammation, University of Leicester, Maurice Shock Building, University Road, Leicester LE1 9HN, UK ([email protected]) Two major types of environment provide habitats for the most xerophilic organisms known: foods pre- served by some form of dehydration or enhanced sugar levels, and hypersaline sites where water availability is limited by a high concentration of salts (usually NaCl). These environments are essentially microbial habitats, with high-sugar foods being dominated by xerophilic (sometimes called osmophilic) filamentous fungi and yeasts, some of which are capable of growth at a water activity (aw) of 0.61, the lowest aw value for growth recorded to date. By contrast, high-salt environments are almost exclusively populated by prokaryotes, notably the haloarchaea, capable of growing in saturated NaCl (aw 0.75). Different strategies are employed for combating the osmotic stress imposed by high levels of solutes in the environment. Eukaryotes and most prokaryotes synthesize or accumulate organic so-called ‘compatible solutes’ (osmolytes) that have counterbalancing osmotic potential. A restricted range of bacteria and the haloar- chaea counterbalance osmotic stress imposed by NaCl by accumulating equivalent amounts of KCl. Haloarchaea become entrapped and survive for long periods inside halite (NaCl) crystals. They are also found in ancient subterranean halite (NaCl) deposits, leading to speculation about survival over geological time periods. Keywords: xerophiles; halophiles; haloarchaea; hypersaline lakes; osmoadaptation; microbial longevity 1. INTRODUCTION aw = P/P0 = n1/n1 ϩ n2, There are two major types of environment in which water where n is moles of solvent (water); n is moles of solute; availability can become limiting for an organism. -

Determination of Hydrolytic Enzyme Capabilities of Halophilic Archaea Isolated from Hides and Skins and Their Phenotypic and Phylogenetic Identification by S
33 DETERMinATION OF HYDROLYTic ENZYME CAPABILITIES OF HALOPHILIC ARCHAEA ISOLATED FROM HIDES AND SKins AND THEIR PHENOTYpic AND PHYLOGENETic IDENTIFicATION by S. T. B LG School of Health, Canakkale Onsekiz Mart University, Terzioglu Campus Canakkale, Turkey, 17100. and B. MER ÇL YaPiCi Biology Department, Faculty of Arts and Science, Canakkale ONSEKIZ MART UNIVERSITY, TERZIOGLU CAMPUS, Canakkale, Turkey, 17100. and İsmail Karaboz Basic and Industrial Microbiology Section, Biology Department, Faculty of Science, Ege University, Bornova, İzmi r, Turkey, 35100. ABSTRACT INTRODUCTION This research aims to isolate extremely halophilic archaea The main constituent of the raw hide is protein, mainly from salted hides, to determine the capacities of their collagen (33% w/w), and remainder is moisture and fat. During hydrolytic enzymes, and to identify them by using phenotypic storage of raw hide, collagen’s excessive proteolysis by and molecular methods. Domestic and imported salted hide lysosomal autolysis or proteolytic bacterial enzymes can lead and skin samples obtained from eight different sources were to the disintegration of the structure of collagen fibers.1 used as the research material. 186 extremely halophilic Biodeterioration is among the major causes of impairment of microorganisms were isolated from salted raw hides and aesthetic, functional and other properties of leather and other skins. Some biochemical, antibiotic sensitivity, pH, NaCl, biopolymers or organic materials and the products made from temperature tolerance and quantitative and qualitative them. Due to the fact that prevention of biological degradation hydrolytic enzyme tests were performed on these isolates. In is very important in conservation and processing of leather, our study, taking into account the phenotypic findings of the great effort is being made for decontamination of these research, 34 of 186 isolates were selected. -

A Grain of Salt
Illinois Wesleyan University Digital Commons @ IWU The Kemp Foundation's Teaching Honorees for Teaching Excellence Excellence Award Ceremony 7-27-2020 A Grain of Salt Timothy Rettich Illinois Wesleyan University Follow this and additional works at: https://digitalcommons.iwu.edu/teaching_excellence Part of the Higher Education Commons Recommended Citation Rettich, Timothy, "A Grain of Salt" (2020). Honorees for Teaching Excellence. 53. https://digitalcommons.iwu.edu/teaching_excellence/53 This Article is protected by copyright and/or related rights. It has been brought to you by Digital Commons @ IWU with permission from the rights-holder(s). You are free to use this material in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This material has been accepted for inclusion by faculty at Illinois Wesleyan University. For more information, please contact [email protected]. ©Copyright is owned by the author of this document. Kemp Award Address 2020 Timothy Rettich “A Grain of Salt” April 8! The theme of this talk was written with this date for Honors Convocation in mind. It was intended to be not only timely, but also somewhat light-hearted. The times have changed, but I decided to keep the same talk, thinking that light-heartedness, like toilet paper, is now in short supply. But by keeping the same talk you will note some anachronisms. Those of you playing along at home can keep track of how many temporal mistakes I make. -

Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea
Hindawi Publishing Corporation Archaea Volume 2011, Article ID 693253, 13 pages doi:10.1155/2011/693253 Review Article Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea Annarita Poli,1 Paola Di Donato,1, 2 Gennaro Roberto Abbamondi,1 and Barbara Nicolaus1 1 Institute of Biomolecular Chemistry (ICB), National Research Council (CNR), Via Campi Flegrei 34, 80078 Pozzuoli, Italy 2 Department of Environmental Sciences, University of Naples “Parthenope”, Centro Direzionale, Isola C4, 80143 Naples, Italy Correspondence should be addressed to Barbara Nicolaus, [email protected] Received 13 May 2011; Accepted 11 July 2011 Academic Editor: Alessandra Morana Copyright © 2011 Annarita Poli et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Extreme environments, generally characterized by atypical temperatures, pH, pressure, salinity, toxicity, and radiation levels, are inhabited by various microorganisms specifically adapted to these particular conditions, called extremophiles. Among these, the microorganisms belonging to the Archaea domain are of significant biotechnological importance as their biopolymers possess unique properties that offer insights into their biology and evolution. Particular attention has been devoted to two main types of biopolymers produced by such peculiar microorganisms, that is, the extracellular polysaccharides (EPSs), considered as a protection against desiccation and predation, and the endocellular polyhydroxyalkanoates (PHAs) that provide an internal reserve of carbon and energy. Here, we report the composition, biosynthesis, and production of EPSs and PHAs by different archaeal species. 1. Introduction that have served as sources for isolation of microbial EPS producers. -

HALOPHILIC MICROORGANISMS and THEIR ENVIRONMENTS Cellular Origin and Life in Extreme Habitats
HALOPHILIC MICROORGANISMS AND THEIR ENVIRONMENTS Cellular Origin and Life in Extreme Habitats Volume 5 Series Editor: Joseph Seckbach Hebrew University of Jerusalem, Israel Halophilic Microorganisms and their Environments by Aharon Oren The Institute of Life Science, The Hebrew University of Jerusalem, Jerusalem, Israel KLUWER ACADEMIC PUBLISHERS NEW YORK, BOSTON, DORDRECHT, LONDON, MOSCOW eBook ISBN: 0-306-48053-0 Print ISBN: 1-4020-0829-5 ©2003 Kluwer Academic Publishers New York, Boston, Dordrecht, London, Moscow Print ©2002 Kluwer Academic Publishers Dordrecht All rights reserved No part of this eBook may be reproduced or transmitted in any form or by any means, electronic, mechanical, recording, or otherwise, without written consent from the Publisher Created in the United States of America Visit Kluwer Online at: http://kluweronline.com and Kluwer's eBookstore at: http://ebooks.kluweronline.com DEDICATION This book is dedicated to the memory of Donn J. Kushner, one of the founding fathers of halophile microbiology, who passed away on September 15, 2001, at the age of 74. My first meeting with Donn took place at the 1983 ASM meeting in New Orleans. At the time I was a newcomer in the field of halophile science, and I clearly remember how thrilled I was to meet one of the 'big' names in the field. It quickly appeared that we shared not only an interest in science, but in music as well. Donn was an accomplished player of the violin and the viola. We have since performed chamber music together at scientific meetings in Jerusalem (1987), in Alicante (1989, together with Morris Kates playing the violin and Masamichi Kohiyama on 'cello), in Williamsburg (1992, again with Morris Kates' violin and Larry Hochstein's clarinet), and once more in Jerusalem (1997, again joined by Larry Hochstein). -

Salt by the Sea
[Salt/Sodium Reduction] Vol. 18 No. 3 March 2008 ww Salt by the Sea By Deb North, Contributing Editor Salt has been in general use before history was even recorded. According to the Salt Institute, Alexandria, VA, about 4,700 years ago, the "Peng-Tzao-Kan-Mu" was published in China, and is regarded as the earliest known treatise on pharmacology emphasizing a discussion of more than 40 kinds of salt. Today, there are hundreds, maybe thousands, of varieties of salt, with some 14,000 known uses. Food-grade salt use accounts for as little as 5%, according to the Salt Institute. (The largest markets are for highway salt and water conditioning.) A grain of salt Salt is the oldest known food additive, and the most widely used of all food preservatives. Sea salt is in a category all its own, distinguished from other varieties such as table, iodized, kosher, rock and pickling salts. "Sea salt is a natural product that does not contain any commercial additives," says Al Kirchner, CEO, Ocean’s Flavor Foods, Asheville, NC, which makes a product that has 45% to 57% less sodium than regular sea salt. Sea salt is derived from living oceans and saline seas and is typically formed in an open environment (usually a shallow pond), with wind, sun and climate conditions that cause evaporation that, in the end, forms crystals. The evaporation process concentrates the sea saltwater, or brine, which is moved pond to pond where heavy deposits of calcium sulfate are laid down. "At certain concentrations or salinities, specific minerals or salts begin to crystallize and recrystallize and drop out of the brine," says George Lutz, quality assurance—technical services manager, Cargill Salt, Minneapolis. -

Biochemical Characterization of the Amylase Activity from the New Haloarchaeal Strain Haloarcula Sp
biology Article Biochemical Characterization of the Amylase Activity from the New Haloarchaeal Strain Haloarcula sp. HS Isolated in the Odiel Marshlands Patricia Gómez-Villegas 1 , Javier Vigara 1 , Luis Romero 2 , Cecilia Gotor 2 , Sara Raposo 3 , Brígida Gonçalves 3 and Rosa Léon 1,* 1 Laboratory of Biochemistry, Department of Chemistry, Marine International Campus of Excellence (CEIMAR), University of Huelva, Avda. de las Fuerzas Armadas s/n, 21071 Huelva, Spain; [email protected] (P.G.-V.); [email protected] (J.V.) 2 Instituto de Bioquímica Vegetal y Fotosíntesis, Consejo Superior de Investigaciones Científicas and Universidad de Sevilla, Avenida Américo Vespucio 49, 41092 Seville, Spain; [email protected] (L.R.); [email protected] (C.G.) 3 CIMA—Centre for Marine and Environmental Research, FCT, Campus de Gambelas, Universidade do Algarve, 8005-139 Faro, Portugal; [email protected] (S.R.); [email protected] (B.G.) * Correspondence: [email protected]; Tel.: +34-959-219-951 Simple Summary: Amylases are a group of enzymes that degrade starch into simple sugars. These proteins are produced by a wide variety of organisms and are supposed to be one of the most valuable industrial enzymes. However, the extreme conditions required for many industrial operations limit the applicability of most amylases found in nature. In this context, halophilic archaea entail an excellent source of novel proteins that tolerate harsh conditions, as they live in environments with Citation: Gómez-Villegas, P.; Vigara, high salt concentration and temperature. In this work, a screening of haloarchaea, isolated from Odiel J.; Romero, L.; Gotor, C.; Raposo, S.; salterns in the southwest of Spain, was carried out to select a new strain with a high amylase activity. -

Carol Litchfield Collection on the History of Salt 2012.219
Carol Litchfield collection on the history of salt 2012.219 This finding aid was produced using ArchivesSpace on September 14, 2021. Description is written in: English. Describing Archives: A Content Standard Audiovisual Collections PO Box 3630 Wilmington, Delaware 19807 [email protected] URL: http://www.hagley.org/library Carol Litchfield collection on the history of salt 2012.219 Table of Contents Summary Information .................................................................................................................................... 7 Historical Note ............................................................................................................................................... 8 Scope and Content ....................................................................................................................................... 11 Administrative Information .......................................................................................................................... 16 Controlled Access Headings ........................................................................................................................ 17 Collection Inventory ..................................................................................................................................... 17 United States .............................................................................................................................................. 17 Alabama ................................................................................................................................................. -

Hydrolytic Enzymes of Halophilic Microorganisms and Their Economic Values
REVIEW ARTICLES HYDROLYTIC ENZYMES OF HALOPHILIC MICROORGANISMS AND THEIR ECONOMIC VALUES MĂDĂLIN ENACHE,1* MASAHIRO KAMEKURA2 1Institute of Biology Bucharest of the Romanian Academy, Splaiul Independentei 296, P.O. Box 56-53, Bucharest, Romania 2Halophiles Research Institute, 677-1 Shimizu, Noda 278-0043, Japan (Received April 1, 2010) Halophilic enzymes, known as extremozymes produced by halophilic microorganisms, have identical enzymatic features like their non-halophilic counterpart, but they exhibit different properties mainly in structure. Among these, two main points could be mentioned, (i) a high content in acidic amino-acids located predominantly at the protein surface and (ii) requirement for high salt concentration for better biological functions. The latter could be understood as a consequence of the abundance of acidic amino-acids but these two fundamental characteristics of halophilic proteins are highly cross-linking each other, making it difficult to explain unambiguously which promotes stability and function at high salinity. The following communication reviews various extracellularly produced hydrolytic enzymes from halophilic microorganisms and their economical values together with basic characteristics of protein chemistry in media with high concentration of sodium chloride and other salts. Key words: halophilic enzyme, extremozyme, halophilic microorganisms, salt, archaea. INTRODUCTION In recent years, investigations to search for biocatalysts that can cope with the conditions of industrial process have been continuously increasing. Organic chemical syntheses are characterized by high precision and purity of products, but they sometimes impose a hazardous effect on the environment. Enzymes, bio- catalysts, on the other hand, often have better chemical precision, which can lead to a more efficient production of single stereoisomer, decreasing the number of secondary reactions, and with a lower environmental impact.