Biodiversity of Archaea and Floral of Two Inland Saltern Ecosystems In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Latest Press Release from Consensus Action on Salt and Health
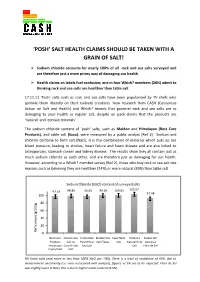
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

1.5. Raman Spectroscopy
Open Research Online The Open University’s repository of research publications and other research outputs Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Thesis How to cite: Rolfe, Samantha (2017). Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration. PhD thesis The Open University. For guidance on citations see FAQs. c 2016 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.0000c66a Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Samantha Melanie Rolfe MPhys (Hons), University of Leicester, 2010 September 2016 The Open University School of Physical Sciences A THESIS SUBMITTED TO THE OPEN UNIVERSITY IN THE SUBJECT OF PLANETARY SCIENCES FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Acknowledgements To my parents, Mark and Melanie, and my brother, Alex, who have always supported and encouraged me to follow my dreams and overcome all and any obstacles to achieve them. To my grandparents, Anthony and Margery Wilson, Aubrey and Val Rolfe and Marjorie and Russell Whitmore, this work is dedicated to you. To Chris, my rock, without you I absolutely would not have been able to get through this process. -

Insights on Cadmium Removal by Bioremediation: the Case of Haloarchaea
Review Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea Mónica Vera-Bernal 1 and Rosa María Martínez-Espinosa 1,2,* 1 Biochemistry and Molecular Biology Division, Agrochemistry and Biochemistry Department, Faculty of Sciences, University of Alicante, Ap. 99, E-03080 Alicante, Spain; [email protected] 2 Multidisciplinary Institute for Environmental Studies “Ramón Margalef”, University of Alicante, Ap. 99, E-03080 Alicante, Spain * Correspondence: [email protected]; Tel.: +34-965903400 (ext. 1258; 8841) Abstract: Although heavy metals are naturally found in the environment as components of the earth’s crust, environmental pollution by these toxic elements has increased since the industrial revolution. Some of them can be considered essential, since they play regulatory roles in different biological processes; but the role of other heavy metals in living tissues is not clear, and once ingested they can accumulate in the organism for long periods of time causing adverse health effects. To mitigate this problem, different methods have been used to remove heavy metals from water and soil, such as chelation-based processes. However, techniques like bioremediation are leaving these conventional methodologies in the background for being more effective and eco-friendlier. Recently, different research lines have been promoted, in which several organisms have been used for bioremediation approaches. Within this context, the extremophilic microorganisms represent one of the best tools for the treatment of contaminated sites due to the biochemical and molecular properties they show. Furthermore, since it is estimated that 5% of industrial effluents are saline and hypersaline, halophilic microorganisms have been suggested as good candidates for bioremediation Citation: Vera-Bernal, M.; and treatment of this kind of samples. -

The Changing Technology of Post Medieval Sea Salt Production in England
1 Heritage, Uses and Representations of the Sea. Centro de Investigação Transdisiplinar Cultura, Espaço e Memoría (CITCEM) Porto, Faculdade de Letras da Universidade do Porto, 20-22 October 2011. The changing technology of post medieval sea salt production in England Jeremy Greenwood Composition of seawater Sea water contains 3.5% evaporites of which salt (sodium chloride) comprises 77.8%. The remainder is known as bittern as it includes the bitter tasting, aperient and deliquescent sulphates of magnesium (Epsom salt) and sodium (Glauber’s salt) as well as about 11% magnesium chloride. 2 Successful commercial salt making depends on the fractional crystallisation of seawater producing the maximum amount of salt without contamination by bittern salts. As seawater is evaporated, very small amounts of calcium carbonate are precipitated followed by some calcium sulphate. This is followed by the crystallisation of sodium chloride but before this is complete, bitter Epsom salt appears; something that needs to be avoided.1 In Continental Europe, evaporation of sea water is achieved solely by the energy of the wind and sun but this is not possible in the English climate so other techniques were developed. 1 http://www.solarsaltharvesters.com/notes.htm SOLAR SALT ENGINEERING 3 Evaporation vessel Briquetage The earliest known English method of coastal saltmaking has been found in the late Bronze Age. This involved boiling seawater in crude clay dishes supported by clay firebars (briquetage) and was widespread in Europe. This technique continued into the Iron Age and into the Roman period with variations inevitably occurring in the industry, although the dating of saltworks is very problematical.2 Detailed interpretation continues to be a matter of dispute. -

Contribution À L'étude Du Genre Tamarix
REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE MINISTERE DE L’ENSEIGNEMENT SUPERIEURET DE LA RECHERCHE SCIENTIFIQUE UNIVERSITE DE TLEMCEN Faculté des Sciences de la Nature etde la Vie et des Sciences de la Terre et de L’Univers Département d’Ecologie et Environnement Laboratoire de recherche d’écologie et gestion des écosystèmes naturels MEMOIREPrésenté par HADJ ALLAL Fatima zahra En vue de l’obtention du Magister En Ecologie Option : Phytodynamique des écosystèmes matorrals menacées Contribution à l'étude du genre Tamarix: aspects botanique et Phyto-écologique dans la région de Tlemcen Présenté le : Président : Mr BOUAZZA Mohamed Professeur Université de Tlemcen Encadreur :Mme STAMBOULI Hassiba M.C.A Université de Tlemcen Examinateurs : Mme MEDJAHDI Assia M.C.A Université de Tlemcen Mr MERZOUK Abdessamad M.C.A Université de Tlemcen Mr HASNAOUI Okkacha M.C.A Université de Saida Invité : Mr HASSANI Fayçal M.C.B Université de Tlemcen Année Universitaire 2013/2014 الملخص: هذهالدراسةمكرسةلدراسةجنساثل،معمراعاةالموكبالنباتيالتيتمتدعلىضفاف "واديتافنا" منحمامبوغرارة،إلىالبحراﻷبيضالمتوسط )راتشجون(. تمالتوصﻹلىالنتائجعلىجنساثلبشكلعام،بمافيذلكالجوانبالبيولوجية،البيوجغرافيواﻹيكولوجية. ويعكسالدراسةالنباتيالقحولةمتسلسلةمنالمنطقةبالحضورالقوىلﻷنواعتتحمﻻلجفافمثلكادرووسأتراكت يليسوبارباتوسشيسموس. خضعتهذهالنظماﻹيكولوجيةضخمةالواجبأساساالتغيراتاﻹنسانومناخالعمل؛تفضلهذاالتطورالتراجعي انتشاربعضاﻷنواعالشوكيةوالسامةالتيتهيمنالواضحلدينامجاﻻلدراسة. الكلماتالرئيسية: واديتافنااثل-متسامح-إيكو- المناخ،ومنطقةالدراسة Résumé : Cette étude est consacrée à l’étude du genre -

Life at Low Water Activity
Published online 12 July 2004 Life at low water activity W. D. Grant Department of Infection, Immunity and Inflammation, University of Leicester, Maurice Shock Building, University Road, Leicester LE1 9HN, UK ([email protected]) Two major types of environment provide habitats for the most xerophilic organisms known: foods pre- served by some form of dehydration or enhanced sugar levels, and hypersaline sites where water availability is limited by a high concentration of salts (usually NaCl). These environments are essentially microbial habitats, with high-sugar foods being dominated by xerophilic (sometimes called osmophilic) filamentous fungi and yeasts, some of which are capable of growth at a water activity (aw) of 0.61, the lowest aw value for growth recorded to date. By contrast, high-salt environments are almost exclusively populated by prokaryotes, notably the haloarchaea, capable of growing in saturated NaCl (aw 0.75). Different strategies are employed for combating the osmotic stress imposed by high levels of solutes in the environment. Eukaryotes and most prokaryotes synthesize or accumulate organic so-called ‘compatible solutes’ (osmolytes) that have counterbalancing osmotic potential. A restricted range of bacteria and the haloar- chaea counterbalance osmotic stress imposed by NaCl by accumulating equivalent amounts of KCl. Haloarchaea become entrapped and survive for long periods inside halite (NaCl) crystals. They are also found in ancient subterranean halite (NaCl) deposits, leading to speculation about survival over geological time periods. Keywords: xerophiles; halophiles; haloarchaea; hypersaline lakes; osmoadaptation; microbial longevity 1. INTRODUCTION aw = P/P0 = n1/n1 ϩ n2, There are two major types of environment in which water where n is moles of solvent (water); n is moles of solute; availability can become limiting for an organism. -

F9.3 Mediterranean Riparian Scrub
European Red List of Habitats - Heathland Habitat Group F9.3 Mediterranean riparian scrub Summary This riparian scrub is an azonal habitat of irregularly flooded environments in a warm Mediterranean climate, occurring mainly along the beds of uncontrolled rivers but also in relatively fresh or brackish coastal sites. Summer drought is long and severe and, even inland, salinisation is possible. The habitat is threatened by changes in the hydrology of rivers to maintain water supplies and also by pollution, afforestation, removal of shrubs for cultivation and intensive grazing and urbanisation. Synthesis The overall decrease in quantity is relatively low (-12%), resulting in the category Least Concern (LC). The quality shows a slight negative trend affecting on average 20% of the surface with a moderately high severity (49%). Also these values lead to the conclusion Least Concern. Overall Category & Criteria EU 28 EU 28+ Red List Category Red List Criteria Red List Category Red List Criteria Least Concern - Least Concern - Sub-habitat types that may require further examination The Mediterranean riparian scrub is defined to include some coastal scrub on the Black Sea shores. This subhabitat differs from the core of the habitat definition, which relates to Mediterranean alluvial scrub, and may be considered as a separate habitat for assessing the threatened status. It is possible (but not certain, because of data gaps) that the Black Sea shore type is more threatened than the Mediterranean type. Habitat Type Code and name F9.3 Mediterranean riparian scrub Mediterranean riparian scrub with Nerium oleander at Fiumara Castelbuono, Nerio oleandri-Salicetum pedicellatae in Andalusia, southern Spain (Photo: Carlos Palermo, Italy (Photo: Ricardo Guarino). -

The Role of Stress Proteins in Haloarchaea and Their Adaptive Response to Environmental Shifts
biomolecules Review The Role of Stress Proteins in Haloarchaea and Their Adaptive Response to Environmental Shifts Laura Matarredona ,Mónica Camacho, Basilio Zafrilla , María-José Bonete and Julia Esclapez * Agrochemistry and Biochemistry Department, Biochemistry and Molecular Biology Area, Faculty of Science, University of Alicante, Ap 99, 03080 Alicante, Spain; [email protected] (L.M.); [email protected] (M.C.); [email protected] (B.Z.); [email protected] (M.-J.B.) * Correspondence: [email protected]; Tel.: +34-965-903-880 Received: 31 July 2020; Accepted: 24 September 2020; Published: 29 September 2020 Abstract: Over the years, in order to survive in their natural environment, microbial communities have acquired adaptations to nonoptimal growth conditions. These shifts are usually related to stress conditions such as low/high solar radiation, extreme temperatures, oxidative stress, pH variations, changes in salinity, or a high concentration of heavy metals. In addition, climate change is resulting in these stress conditions becoming more significant due to the frequency and intensity of extreme weather events. The most relevant damaging effect of these stressors is protein denaturation. To cope with this effect, organisms have developed different mechanisms, wherein the stress genes play an important role in deciding which of them survive. Each organism has different responses that involve the activation of many genes and molecules as well as downregulation of other genes and pathways. Focused on salinity stress, the archaeal domain encompasses the most significant extremophiles living in high-salinity environments. To have the capacity to withstand this high salinity without losing protein structure and function, the microorganisms have distinct adaptations. -

Characterization of the Microbial Population Inhabiting a Solar Saltern Pond of the Odiel Marshlands (SW Spain)
marine drugs Article Characterization of the Microbial Population Inhabiting a Solar Saltern Pond of the Odiel Marshlands (SW Spain) Patricia Gómez-Villegas, Javier Vigara and Rosa León * Laboratory of Biochemistry and Molecular Biology, Faculty of Experimental Sciences, Marine International Campus of Excellence (CEIMAR), University of Huelva, 21071 Huelva, Spain; [email protected] (P.G.-V.); [email protected] (J.V.) * Correspondence: [email protected]; Tel.: +34-959-219-951 Received: 28 June 2018; Accepted: 8 September 2018; Published: 12 September 2018 Abstract: The solar salterns located in the Odiel marshlands, in southwest Spain, are an excellent example of a hypersaline environment inhabited by microbial populations specialized in thriving under conditions of high salinity, which remains poorly explored. Traditional culture-dependent taxonomic studies have usually under-estimated the biodiversity in saline environments due to the difficulties that many of these species have to grow at laboratory conditions. Here we compare two molecular methods to profile the microbial population present in the Odiel saltern hypersaline water ponds (33% salinity). On the one hand, the construction and characterization of two clone PCR amplified-16S rRNA libraries, and on the other, a high throughput 16S rRNA sequencing approach based on the Illumina MiSeq platform. The results reveal that both methods are comparable for the estimation of major genera, although massive sequencing provides more information about the less abundant ones. The obtained data indicate that Salinibacter ruber is the most abundant genus, followed by the archaea genera, Halorubrum and Haloquadratum. However, more than 100 additional species can be detected by Next Generation Sequencing (NGS). In addition, a preliminary study to test the biotechnological applications of this microbial population, based on its ability to produce and excrete haloenzymes, is shown. -

CRMS Vegetation Analytical Team Framework: Methods for Collection, Development, and Use of Vegetation Response Variables
CRMS Vegetation Analytical Team Framework: Methods for Collection, Development, and Use of Vegetation Response Variables By Kari F. Cretini, Jenneke M. Visser, Ken W. Krauss, and Gregory D. Steyer Open-File Report 2011–1097 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia 2011 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested citation: Cretini, K.F., Visser, J.M., Krauss, K.W.,and Steyer, G.D., 2011, CRMS vegetation analytical team framework—Methods for collection, development, and use of vegetation response variables: U.S. Geological Survey Open-File Report 2011-1097, 60 p. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. ii Contents Abstract ............................................................................................................................................. 1 Introduction ...................................................................................................................................... -

Determination of Hydrolytic Enzyme Capabilities of Halophilic Archaea Isolated from Hides and Skins and Their Phenotypic and Phylogenetic Identification by S
33 DETERMinATION OF HYDROLYTic ENZYME CAPABILITIES OF HALOPHILIC ARCHAEA ISOLATED FROM HIDES AND SKins AND THEIR PHENOTYpic AND PHYLOGENETic IDENTIFicATION by S. T. B LG School of Health, Canakkale Onsekiz Mart University, Terzioglu Campus Canakkale, Turkey, 17100. and B. MER ÇL YaPiCi Biology Department, Faculty of Arts and Science, Canakkale ONSEKIZ MART UNIVERSITY, TERZIOGLU CAMPUS, Canakkale, Turkey, 17100. and İsmail Karaboz Basic and Industrial Microbiology Section, Biology Department, Faculty of Science, Ege University, Bornova, İzmi r, Turkey, 35100. ABSTRACT INTRODUCTION This research aims to isolate extremely halophilic archaea The main constituent of the raw hide is protein, mainly from salted hides, to determine the capacities of their collagen (33% w/w), and remainder is moisture and fat. During hydrolytic enzymes, and to identify them by using phenotypic storage of raw hide, collagen’s excessive proteolysis by and molecular methods. Domestic and imported salted hide lysosomal autolysis or proteolytic bacterial enzymes can lead and skin samples obtained from eight different sources were to the disintegration of the structure of collagen fibers.1 used as the research material. 186 extremely halophilic Biodeterioration is among the major causes of impairment of microorganisms were isolated from salted raw hides and aesthetic, functional and other properties of leather and other skins. Some biochemical, antibiotic sensitivity, pH, NaCl, biopolymers or organic materials and the products made from temperature tolerance and quantitative and qualitative them. Due to the fact that prevention of biological degradation hydrolytic enzyme tests were performed on these isolates. In is very important in conservation and processing of leather, our study, taking into account the phenotypic findings of the great effort is being made for decontamination of these research, 34 of 186 isolates were selected. -

Warren, J. K., 2010, Evaporites Through Time: Tectonic, Climatic And
Earth-Science Reviews 98 (2010) 217–268 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Evaporites through time: Tectonic, climatic and eustatic controls in marine and nonmarine deposits John K. Warren Petroleum Geoscience Program, Department of Geology, Chulalongkorn University, 254 Phayathai Road, Pathumwan, Bangkok 10330, Thailand article info abstract Article history: Throughout geological time, evaporite sediments form by solar-driven concentration of a surface or Received 25 February 2009 nearsurface brine. Large, thick and extensive deposits dominated by rock-salt (mega-halite) or anhydrite Accepted 10 November 2009 (mega-sulfate) deposits tend to be marine evaporites and can be associated with extensive deposits of Available online 22 November 2009 potash salts (mega-potash). Ancient marine evaporite deposition required particular climatic, eustatic or tectonic juxtapositions that have occurred a number of times in the past and will so again in the future. Keywords: Ancient marine evaporites typically have poorly developed Quaternary counterparts in scale, thickness, evaporite deposition tectonics and hydrology. When mega-evaporite settings were active within appropriate arid climatic and marine hydrological settings then huge volumes of seawater were drawn into the subsealevel evaporitic nonmarine depressions. These systems were typical of regions where the evaporation rates of ocean waters were at plate tectonics their maximum, and so were centred on the past latitudinal equivalents of today's horse latitudes. But, like economic geology today's nonmarine evaporites, the location of marine Phanerozoic evaporites in zones of appropriate classification adiabatic aridity and continentality extended well into the equatorial belts. Exploited deposits of borate, sodium carbonate (soda-ash) and sodium sulfate (salt-cake) salts, along with evaporitic sediments hosting lithium-rich brines require continental–meteoric not marine-fed hydrologies.