Life at Low Water Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
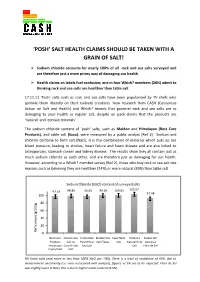
Latest Press Release from Consensus Action on Salt and Health
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

Extremozymes of the Hot and Salty Halothermothrix Orenii
Extremozymes of the Hot and Salty Halothermothrix orenii Author Kori, Lokesh D Published 2012 Thesis Type Thesis (PhD Doctorate) School School of Biomolecular and Physical Sciences DOI https://doi.org/10.25904/1912/2191 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/366220 Griffith Research Online https://research-repository.griffith.edu.au Extremozymes of the hot and salty Halothermothrix orenii LOKESH D. KORI (M.Sc. Biotechnology) School of Biomolecular and Physical Sciences Science, Environment, Engineering and Technology Griffith University, Australia Submitted in fulfillment of the requirements of the degree of Doctor of Philosophy December 2011 STATEMENT OF ORIGINALITY STATEMENT OF ORIGINALITY This work has not previously been submitted for a degree or diploma in any university. To the best of my knowledge and belief, the thesis contains no material previously published or written by another person except where due reference is made in the thesis itself. LOKESH DULICHAND KORI II ACKNOWLEDGEMENTS ACKNOWLEDGEMENTS I owe my deepest gratitude to my supervisor Prof. Bharat Patel, for offering me an opportunity for being his postgraduate. His boundless knowledge motivates me for keep going and enjoy the essence of science. Without his guidance, great patience and advice, I could not finish my PhD program successfully. I take this opportunity to give my heartiest thanks to Assoc. Prof. Andreas Hofmann, (Structural Chemistry, Eskitis Institute for Cell & Molecular Therapies, Griffith University) for his support and encouragement for crystallographic work. I am grateful to him for teaching me about the protein structures, in silico analysis and their hidden chemistry. -

1.5. Raman Spectroscopy
Open Research Online The Open University’s repository of research publications and other research outputs Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Thesis How to cite: Rolfe, Samantha (2017). Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration. PhD thesis The Open University. For guidance on citations see FAQs. c 2016 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.0000c66a Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Samantha Melanie Rolfe MPhys (Hons), University of Leicester, 2010 September 2016 The Open University School of Physical Sciences A THESIS SUBMITTED TO THE OPEN UNIVERSITY IN THE SUBJECT OF PLANETARY SCIENCES FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Acknowledgements To my parents, Mark and Melanie, and my brother, Alex, who have always supported and encouraged me to follow my dreams and overcome all and any obstacles to achieve them. To my grandparents, Anthony and Margery Wilson, Aubrey and Val Rolfe and Marjorie and Russell Whitmore, this work is dedicated to you. To Chris, my rock, without you I absolutely would not have been able to get through this process. -

Insights on Cadmium Removal by Bioremediation: the Case of Haloarchaea
Review Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea Mónica Vera-Bernal 1 and Rosa María Martínez-Espinosa 1,2,* 1 Biochemistry and Molecular Biology Division, Agrochemistry and Biochemistry Department, Faculty of Sciences, University of Alicante, Ap. 99, E-03080 Alicante, Spain; [email protected] 2 Multidisciplinary Institute for Environmental Studies “Ramón Margalef”, University of Alicante, Ap. 99, E-03080 Alicante, Spain * Correspondence: [email protected]; Tel.: +34-965903400 (ext. 1258; 8841) Abstract: Although heavy metals are naturally found in the environment as components of the earth’s crust, environmental pollution by these toxic elements has increased since the industrial revolution. Some of them can be considered essential, since they play regulatory roles in different biological processes; but the role of other heavy metals in living tissues is not clear, and once ingested they can accumulate in the organism for long periods of time causing adverse health effects. To mitigate this problem, different methods have been used to remove heavy metals from water and soil, such as chelation-based processes. However, techniques like bioremediation are leaving these conventional methodologies in the background for being more effective and eco-friendlier. Recently, different research lines have been promoted, in which several organisms have been used for bioremediation approaches. Within this context, the extremophilic microorganisms represent one of the best tools for the treatment of contaminated sites due to the biochemical and molecular properties they show. Furthermore, since it is estimated that 5% of industrial effluents are saline and hypersaline, halophilic microorganisms have been suggested as good candidates for bioremediation Citation: Vera-Bernal, M.; and treatment of this kind of samples. -

Salt Deposits in the UK
CORE Metadata, citation and similar papers at core.ac.uk Provided by NERC Open Research Archive Halite karst geohazards (natural and man-made) in the United Kingdom ANTHONY H. COOPER British Geological Survey, Keyworth, Nottingham, NG12 5GG, Great Britain COPYRIGHT © BGS/NERC e-mail [email protected] +44 (-0)115 936 3393 +44 (-0)115 936 3475 COOPER, A.H. 2002. Halite karst geohazards (natural and man-made) in the United Kingdom. Environmental Geology, Vol. 42, 505-512. This work is based on a paper presented to the 8th Multidisciplinary Conference on Sinkholes and the Engineering and Environmental impact of karst, Louisville, Kentucky, April 2001. In the United Kingdom Permian and Triassic halite (rock salt) deposits have been affected by natural and artificial dissolution producing karstic landforms and subsidence. Brine springs from the Triassic salt have been exploited since Roman times, or possibly earlier, indicating prolonged natural dissolution. Medieval salt extraction in England is indicated by the of place names ending in “wich” indicating brine spring exploitation at Northwich, Middlewich, Nantwich and Droitwich. Later, Victorian brine extraction in these areas accentuated salt karst development causing severe subsidence problems that remain a legacy. The salt was also mined, but the mines flooded and consequent brine extraction caused the workings to collapse, resulting in catastrophic surface subsidence. Legislation was enacted to pay for the damage and a levy is still charged for salt extraction. Some salt mines are still collapsing and the re-establishment of the post-brine extraction hydrogeological regimes means that salt springs may again flow causing further dissolution and potential collapse. -

About the Urban Development of Hallstatt
About the Urban Development of Hallstatt CONTENTS 1. STARTING POINT 2 2. TOPOGRAPHY 3 2.1. Topographical survey 3 The Lake 3 The Mountains 3 The Communes of the Lake of Hallstatt 3 The Market Commune of Hallstatt 3 Names of Streets, Meadows and Marshes 4 2.3. The Morphology of the Villages 4 The Market 4 Lahn 4 3. URBAN DEVELOPMENT 6 3.1 The Development of the Traffic Network 6 The Waterway 6 The Paths 6 The Street 7 The Railway 7 The Pipeline, the so called "Sulzstrenn" 7 3.2. The Evolution of the Buildings 9 3.2.1. The Market 9 The Early History and the Age of Romans 9 The Founding in the Middle Ages 9 The Extension in the 16th Century 9 The Stagnation since the Beginning of the 17th Century until the catastrophic conflagration in 175010 The "Amthof" 10 The Court Chapel 11 The Hospital 11 The Hospital Chapel 11 The "Pfannhaus" and the "Pfieseln" 11 The Recession, Starting in 1750 until the Beginning of Tourism 11 The Flourishing of Tourism 12 3.2.2. Lahn 12 The Roman Ages 12 The Modern Times 12 3.3. The Analysis of the Sites and the Structures 15 3.3.1. The Market 15 The Market Place 16 The Landing Place 16 3.3.2. Lahn 17 The Agricultural Area 17 The Industrial Area 17 The Expansion 17 The Condensing 18 4. ANHANG 19 4.1. Commissions Relation dieses hochen Mittels Hoff Raths Herrn v. Quiex die zu Haalstatt abgebrunnenen Sallz Pfannen betreffend. 19 5. -

LAS VEGAS PRODUCT CATALOG INGREDIENTS Full Page Ad for FINE PASTRY 11”X 8.5”
PRODUCT CATALOG LAS VEGAS chefswarehouse.com BAKING AND PASTRY FROZEN/RTB BREAD ...................12 BEVERAGES, GOAT CHEESE ............................21 CONDIMENTS BAKING JAM ..............................4 PIZZA SHELLS ...............................12 COFFEE AND TEA GOUDA.......................................21 AND JAMS TORTILLAS/WRAPS ......................12 HAVARTI.......................................22 BAKING MIXES ............................4 BAR MIXERS ................................17 CHUTNEY ....................................25 WRAPPERS ..................................12 JACK CHEESE .............................22 BAKING SUPPLIES .......................4 BITTERS .........................................17 GLAZES AND DEMI-GLAZES .......25 BROWNIES ..................................12 MASCARPONE ...........................22 COLORANTS ...............................4 CORDIAL ....................................17 KETCHUP .....................................25 CAKES ASSORTED ......................12 MISCELLANEOUS ........................22 CROISSANTS ...............................4 JUICE ...........................................17 MAYO ..........................................25 TARTS ...........................................13 MOUNTAIN STYLE ........................22 DÉCOR ........................................4 MISCELLANEOUS ........................17 MUSTARD ....................................25 COULIS ........................................13 MOZZARELLA ..............................22 EXTRACTS ....................................6 -

Diversity of Halophilic Archaea in Fermented Foods and Human Intestines and Their Application Han-Seung Lee1,2*
J. Microbiol. Biotechnol. (2013), 23(12), 1645–1653 http://dx.doi.org/10.4014/jmb.1308.08015 Research Article Minireview jmb Diversity of Halophilic Archaea in Fermented Foods and Human Intestines and Their Application Han-Seung Lee1,2* 1Department of Bio-Food Materials, College of Medical and Life Sciences, Silla University, Busan 617-736, Republic of Korea 2Research Center for Extremophiles, Silla University, Busan 617-736, Republic of Korea Received: August 8, 2013 Revised: September 6, 2013 Archaea are prokaryotic organisms distinct from bacteria in the structural and molecular Accepted: September 9, 2013 biological sense, and these microorganisms are known to thrive mostly at extreme environments. In particular, most studies on halophilic archaea have been focused on environmental and ecological researches. However, new species of halophilic archaea are First published online being isolated and identified from high salt-fermented foods consumed by humans, and it has September 10, 2013 been found that various types of halophilic archaea exist in food products by culture- *Corresponding author independent molecular biological methods. In addition, even if the numbers are not quite Phone: +82-51-999-6308; high, DNAs of various halophilic archaea are being detected in human intestines and much Fax: +82-51-999-5458; interest is given to their possible roles. This review aims to summarize the types and E-mail: [email protected] characteristics of halophilic archaea reported to be present in foods and human intestines and pISSN 1017-7825, eISSN 1738-8872 to discuss their application as well. Copyright© 2013 by The Korean Society for Microbiology Keywords: Halophilic archaea, fermented foods, microbiome, human intestine, Halorubrum and Biotechnology Introduction Depending on the optimal salt concentration needed for the growth of strains, halophilic microorganisms can be Archaea refer to prokaryotes that used to be categorized classified as halotolerant (~0.3 M), halophilic (0.2~2.0 M), as archaeabacteria, a type of bacteria, in the past. -

Halophilic Microorganisms Springer-Verlag Berlin Heidelberg Gmbh Antonio Ventosa (Ed.)
Halophilic Microorganisms Springer-Verlag Berlin Heidelberg GmbH Antonio Ventosa (Ed.) Halophilic Microorganisms With 58 Figures Springer Dr.ANTONIO VENTOSA Department of Microbiology and Parasitology Faculty of Pharmacy University of Sevilla 41012 Sevilla Spain e-mail: [email protected] ISBN 978-3-642-05664-2 ISBN 978-3-662-07656-9 (eBook) DOI 10.1007/978-3-662-07656-9 Library of Congress Cataloging-in-Publication Data applied for A catalog record for this book is available from the Library of Congress. Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publicati an in the Deutsche Nationalbibliographie; detailed bibliographic data is available in the Internet at http://dnb.ddb.de This work is subject ta copyright. AII rights are reservod, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of iIIustrations, recitation, broadcasting, reproduction an microfilm ar in any other way, and storage in data banks. Duplication of this publication ar parts thereofis permit ted only under the provisions of the German Copyright Law of September 9, 1965, in ils current version, and per missions for use must always be obtained from Springcr-Vcrlag Berlin Heiddhcrg GmbH. Violations are liable for prosecution under the German Copyright Law. http://www.springer.de © Springer-Verlag Berlin Heidelberg 2004 Originally published by Springer-Vcrlag lkrlin Hcidclbcrg New York in 2004 The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Study of Psychrophilic, Mesophilic, and Halophilic Bacteria in Salt and Meat Curing Solutions
A STUDY OF PSYCHROPHILIC, ilESOPHILIC, AND HALOPHTLIC BACTERIA IN SALT AND MEAT CURING SOLUTIONS by HAROLD EUGENE TICKNER a B, S,, Kansas State College of Agriculture and Applied Science, 1949 A THESIS - submitted in partial fulfillment of the 1 ' r requirements for the degree MASTER OF SCIENCE 1 Department of Bacteriology KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE 1950 X Docu- M TABLE OF CONTENTS \^50 T5 INTRODUCTIi. ^» « • 1 REVIEW OF LITERATURE 2 EXPERIMENT I - PREPARATION OF MEDIA FOR PLATE COUNTS . 17 EXPERIMENT II - PLATE COUNTS FOR ENUMERATION OF RALOPHILIC ORGANISMS IN COMMERCIAL SALT SAMPLES 19 EXPERIMENT III - ISOLATION OF PSYCHROPHILIC- AND MESOPHILIC-HALOPHILIC BACTERIA 21 EXPERIMENT IV - PLATE COUNTS OF CURING SOLUTIONS ... 26 EXPERIMENT V - DETERMINATION OF NUMBERS OF PSYCHROPHILIC ORGANISMS IN THE CURING SOLUTIONS ...... 2fi EXPERIMENT VI - TESTING PURE CULTURES OF PSYCHROPHILIC ORGANISMS IN STERILE CURING SOLUTIONS 33 EXPERIMENT VII - PREPARATION OF CURING SOLUTION TO CHECK GROWTH OF PSYCHROPHILIC- AND MESOPHILIC-HALOPHILIC BACTERIA IN A NEW CURING SOLUTION AO EXPERIMENT VIII - DETERMINATION OF NUMBERS OF MESOPHILIC AND MESOPHILIC-HALOPHILIC BACTERIA IN MEAT CURING SOLUTIONS A2 EXPERIMENT IX - DETERMINATION OF CHLORIDE CONTENT IN OLD USED CURING SOLUTIONS U EXPERIMENT X - DETERMINATION OF AMOUNT OF PROTEINS IN OLD USED CURING SOLUTIONS 46 EXPERIMENT XI - PREPARATION OF A "SYNTHETIC AGED CURING SOLUTION" EMPLOYED AS A MEDIUM FOR CHECKING GROWTH OF PURE CULTURES OF ORGANISMS ISOLATED FROM SALT AND MEAT CURING SOLUTIONS A3 EXPERIMENT XII - A COMPARISON OF BACTERIA FOUND IN SALT AND MEAT CURING SOLUTIONS 55 EXPERIMENT XIII - ORGANISMS REQUIRING SODIUM CHLORIDE FOR GROWTH 57 DISCUSSION 59 ii CONCLUSIONS 64. -

Marine Extremophiles: a Source of Hydrolases for Biotechnological Applications
Mar. Drugs 2015, 13, 1925-1965; doi:10.3390/md13041925 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Article Marine Extremophiles: A Source of Hydrolases for Biotechnological Applications Gabriel Zamith Leal Dalmaso 1,2, Davis Ferreira 3 and Alane Beatriz Vermelho 1,* 1 BIOINOVAR—Biotechnology laboratories: Biocatalysis, Bioproducts and Bioenergy, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] 2 Graduate Program in Plant Biotechnology, Health and Science Centre, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil 3 BIOINOVAR—Biotechnology Laboratories: Virus-Cell Interaction, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-(21)-3936-6743; Fax: +55-(21)-2560-8344. Academic Editor: Kirk Gustafson Received: 1 December 2014 / Accepted: 25 March 2015 / Published: 3 April 2015 Abstract: The marine environment covers almost three quarters of the planet and is where evolution took its first steps. Extremophile microorganisms are found in several extreme marine environments, such as hydrothermal vents, hot springs, salty lakes and deep-sea floors. The ability of these microorganisms to support extremes of temperature, salinity and pressure demonstrates their great potential for biotechnological processes. Hydrolases including amylases, cellulases, peptidases and lipases from hyperthermophiles, psychrophiles, halophiles and piezophiles have been investigated for these reasons. -

Halanaerobaculum Jean Luc Cayol
Halanaerobaculum Jean Luc Cayol To cite this version: Jean Luc Cayol. Halanaerobaculum. Bergey’s Manual of Systematics of Archaea and Bacteria (BMSAB), Hoboken, New Jersey : Wiley, [2015]-, 2019, 10.1002/9781118960608.gbm01725. hal- 02883868 HAL Id: hal-02883868 https://hal.archives-ouvertes.fr/hal-02883868 Submitted on 29 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 gbm01725 2 3 Halanaerobaculum 4 5 Hedi, Fardeau, Sadfi, Boudabous, Ollivier and Cayol 2009, 317 VP 6 7 Jean-Luc Cayol 8 9 Aix Marseille Université, IRD, Université de Toulon, CNRS, Mediterranean Institute of 10 Oceanography (MIO), UM 110, 13288 Marseille cedex 9, France 11 12 Hal.an.ae.ro.ba’cu.lum. Gr. masc. n. hals, salt; Gr. pref. an, not; Gr. masc. or fem. n. aer, air; 13 L. neut. n. baculum, stick; N.L. neut. n. Halanaerobaculum, salt stick not living in air. 14 15 Abstract 16 The genus Halanaerobaculum comprises one species with validly published name, 17 Halanaerobaculum tunisiense, which was isolated from hypersaline surface sediments of El- 18 Djerid Chott, Tunisia. The genus is halophilic, strict anaerobic and chemoorganotrophic. The 19 genus Halanaerobaculum belongs to the family Halobacteroidaceae; order Halanaerobiales; 20 class Clostridia.