Analysis of Haloferax Mediterranei Lrp Transcriptional Regulator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
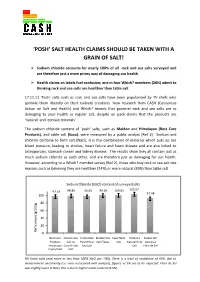
Latest Press Release from Consensus Action on Salt and Health
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

1.5. Raman Spectroscopy
Open Research Online The Open University’s repository of research publications and other research outputs Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Thesis How to cite: Rolfe, Samantha (2017). Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration. PhD thesis The Open University. For guidance on citations see FAQs. c 2016 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.0000c66a Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Characteristic Raman Bands of Amino Acids and Halophiles as Biomarkers in Planetary Exploration Samantha Melanie Rolfe MPhys (Hons), University of Leicester, 2010 September 2016 The Open University School of Physical Sciences A THESIS SUBMITTED TO THE OPEN UNIVERSITY IN THE SUBJECT OF PLANETARY SCIENCES FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Acknowledgements To my parents, Mark and Melanie, and my brother, Alex, who have always supported and encouraged me to follow my dreams and overcome all and any obstacles to achieve them. To my grandparents, Anthony and Margery Wilson, Aubrey and Val Rolfe and Marjorie and Russell Whitmore, this work is dedicated to you. To Chris, my rock, without you I absolutely would not have been able to get through this process. -

Delft University of Technology Halococcoides Cellulosivorans Gen
Delft University of Technology Halococcoides cellulosivorans gen. nov., sp. nov., an extremely halophilic cellulose- utilizing haloarchaeon from hypersaline lakes Sorokin, Dimitry Y.; Khijniak, Tatiana V.; Elcheninov, Alexander G.; Toshchakov, Stepan V.; Kostrikina, Nadezhda A.; Bale, Nicole J.; Sinninghe Damsté, Jaap S.; Kublanov, Ilya V. DOI 10.1099/ijsem.0.003312 Publication date 2019 Document Version Accepted author manuscript Published in International Journal of Systematic and Evolutionary Microbiology Citation (APA) Sorokin, D. Y., Khijniak, T. V., Elcheninov, A. G., Toshchakov, S. V., Kostrikina, N. A., Bale, N. J., Sinninghe Damsté, J. S., & Kublanov, I. V. (2019). Halococcoides cellulosivorans gen. nov., sp. nov., an extremely halophilic cellulose-utilizing haloarchaeon from hypersaline lakes. International Journal of Systematic and Evolutionary Microbiology, 69(5), 1327-1335. [003312]. https://doi.org/10.1099/ijsem.0.003312 Important note To cite this publication, please use the final published version (if applicable). Please check the document version above. Copyright Other than for strictly personal use, it is not permitted to download, forward or distribute the text or part of it, without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license such as Creative Commons. Takedown policy Please contact us and provide details if you believe this document breaches copyrights. We will remove access to the work immediately and investigate your claim. This work is downloaded from Delft University of Technology. For technical reasons the number of authors shown on this cover page is limited to a maximum of 10. International Journal of Systematic and Evolutionary Microbiology Halococcoides cellulosivorans gen. -

Insights on Cadmium Removal by Bioremediation: the Case of Haloarchaea
Review Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea Mónica Vera-Bernal 1 and Rosa María Martínez-Espinosa 1,2,* 1 Biochemistry and Molecular Biology Division, Agrochemistry and Biochemistry Department, Faculty of Sciences, University of Alicante, Ap. 99, E-03080 Alicante, Spain; [email protected] 2 Multidisciplinary Institute for Environmental Studies “Ramón Margalef”, University of Alicante, Ap. 99, E-03080 Alicante, Spain * Correspondence: [email protected]; Tel.: +34-965903400 (ext. 1258; 8841) Abstract: Although heavy metals are naturally found in the environment as components of the earth’s crust, environmental pollution by these toxic elements has increased since the industrial revolution. Some of them can be considered essential, since they play regulatory roles in different biological processes; but the role of other heavy metals in living tissues is not clear, and once ingested they can accumulate in the organism for long periods of time causing adverse health effects. To mitigate this problem, different methods have been used to remove heavy metals from water and soil, such as chelation-based processes. However, techniques like bioremediation are leaving these conventional methodologies in the background for being more effective and eco-friendlier. Recently, different research lines have been promoted, in which several organisms have been used for bioremediation approaches. Within this context, the extremophilic microorganisms represent one of the best tools for the treatment of contaminated sites due to the biochemical and molecular properties they show. Furthermore, since it is estimated that 5% of industrial effluents are saline and hypersaline, halophilic microorganisms have been suggested as good candidates for bioremediation Citation: Vera-Bernal, M.; and treatment of this kind of samples. -

Realizing the Allosteric Potential of the Tetrameric Protein Kinase a RIΑ Holoenzyme
Structure Article Realizing the Allosteric Potential of the Tetrameric Protein Kinase A RIa Holoenzyme Angela J. Boettcher,1,6 Jian Wu,1,6 Choel Kim,2 Jie Yang,1 Jessica Bruystens,1 Nikki Cheung,1 Juniper K. Pennypacker,1,3 Donald A. Blumenthal,4 Alexandr P. Kornev,3,5 and Susan S. Taylor1,3,5,* 1Department of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093, USA 2Department of Pharmacology, Baylor College of Medicine, Houston, TX 77030, USA 3Department of Pharmacology, University of California at San Diego, La Jolla, CA 92093, USA 4Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT 84112, USA 5Howard Hughes Medical Institute, University of California at San Diego, La Jolla, CA 92093, USA 6These authors contributed equally to this work *Correspondence: [email protected] DOI 10.1016/j.str.2010.12.005 SUMMARY the active site cleft in the C subunit in the inactive holoenzyme but is disordered in the dissociated free R subunits (Li et al., PKA holoenzymes containing two catalytic (C) 2000). The linker, as summarized in Figure 1, can be divided subunits and a regulatory (R) subunit dimer are acti- into three segments, the consensus inhibitor site (P-3 to P+1), vated cooperatively by cAMP. While cooperativity the N-linker that joins the inhibitor site to the D/D domain, and involves the two tandem cAMP binding domains in the C-linker that becomes ordered in the heterodimeric holoen- each R-subunit, additional cooperativity is associ- zyme complex. While much has been learned from the structures ated with the tetramer. -

A Perspective on Mechanisms of Protein Tetramer Formation
Biophysical Journal Volume 85 December 2003 3587–3599 3587 A Perspective on Mechanisms of Protein Tetramer Formation Evan T. Powers* and David L. Powersy *Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037; and yDepartment of Mathematics and Computer Science, Clarkson University, Potsdam, New York 13699 ABSTRACT Homotetrameric proteins can assemble by several different pathways, but have only been observed to use one, in which two monomers associate to form a homodimer, and then two homodimers associate to form a homotetramer. To determine why this pathway should be so uniformly dominant, we have modeled the kinetics of tetramerization for the possible pathways as a function of the rate constants for each step. We have found that competition with the other pathways, in which homotetramers can be formed either by the association of two different types of homodimers or by the successive addition of monomers to homodimers and homotrimers, can cause substantial amounts of protein to be trapped as intermediates of the assembly pathway. We propose that this could lead to undesirable consequences for an organism, and that selective pressure may have caused homotetrameric proteins to evolve to assemble by a single pathway. INTRODUCTION Many proteins must be homotetrameric to be functional. ‘‘MDT’’ stands for monomer-dimer-tetramer and ‘‘a’’ and Prominent examples include transcription factors (e.g., p53) ‘‘b’’ indicate the type of homodimer formed. The pathways (Friedman et al., 1993), transport proteins (e.g., trans- that include monomers, homodimers, homotrimers, and thyretin) (Blake et al., 1974), potassium channels (Deutsch, homotetramers will be denoted MDRT, which stands for 2002; Miller, 2000), water channels (Fujiyoshi et al., 2002), monomer-dimer-trimer-tetramer. -

HOOK™ Maleimide Activated Streptavidin for Conjugation of Streptavidin to Sulfhydryl Groups Containing Proteins, Peptides and Ligands
G-Biosciences 1-800-628-7730 1-314-991-6034 [email protected] A Geno Technology, Inc. (USA) brand name HOOK™ Maleimide Activated Streptavidin For conjugation of Streptavidin to sulfhydryl groups containing proteins, peptides and ligands (Cat. #786-1653, 786-1654) think proteins! think G-Biosciences www.GBiosciences.com INTRODUCTION ................................................................................................................. 3 ITEMS SUPPLIED ................................................................................................................ 4 STORAGE CONDITIONS ...................................................................................................... 4 ADDITIONAL ITEMS NEEDED .............................................................................................. 4 IMPORTANT INFORMATION .............................................................................................. 4 PROTOCOL ......................................................................................................................... 4 PREPARATION OF PROTEIN FOR CONJUGATION TO MALEIMIDE ACTIVATED PROTEIN 4 CONJUGATION REACTION ............................................................................................. 5 STORAGE OF CONJUGATED ANTIBODIES/PROTEINS ......................................................... 5 RELATED PRODUCTS .......................................................................................................... 5 Page 2 of 6 INTRODUCTION Streptavidin is a non-glycosylated -

Table S4. Phylogenetic Distribution of Bacterial and Archaea Genomes in Groups A, B, C, D, and X
Table S4. Phylogenetic distribution of bacterial and archaea genomes in groups A, B, C, D, and X. Group A a: Total number of genomes in the taxon b: Number of group A genomes in the taxon c: Percentage of group A genomes in the taxon a b c cellular organisms 5007 2974 59.4 |__ Bacteria 4769 2935 61.5 | |__ Proteobacteria 1854 1570 84.7 | | |__ Gammaproteobacteria 711 631 88.7 | | | |__ Enterobacterales 112 97 86.6 | | | | |__ Enterobacteriaceae 41 32 78.0 | | | | | |__ unclassified Enterobacteriaceae 13 7 53.8 | | | | |__ Erwiniaceae 30 28 93.3 | | | | | |__ Erwinia 10 10 100.0 | | | | | |__ Buchnera 8 8 100.0 | | | | | | |__ Buchnera aphidicola 8 8 100.0 | | | | | |__ Pantoea 8 8 100.0 | | | | |__ Yersiniaceae 14 14 100.0 | | | | | |__ Serratia 8 8 100.0 | | | | |__ Morganellaceae 13 10 76.9 | | | | |__ Pectobacteriaceae 8 8 100.0 | | | |__ Alteromonadales 94 94 100.0 | | | | |__ Alteromonadaceae 34 34 100.0 | | | | | |__ Marinobacter 12 12 100.0 | | | | |__ Shewanellaceae 17 17 100.0 | | | | | |__ Shewanella 17 17 100.0 | | | | |__ Pseudoalteromonadaceae 16 16 100.0 | | | | | |__ Pseudoalteromonas 15 15 100.0 | | | | |__ Idiomarinaceae 9 9 100.0 | | | | | |__ Idiomarina 9 9 100.0 | | | | |__ Colwelliaceae 6 6 100.0 | | | |__ Pseudomonadales 81 81 100.0 | | | | |__ Moraxellaceae 41 41 100.0 | | | | | |__ Acinetobacter 25 25 100.0 | | | | | |__ Psychrobacter 8 8 100.0 | | | | | |__ Moraxella 6 6 100.0 | | | | |__ Pseudomonadaceae 40 40 100.0 | | | | | |__ Pseudomonas 38 38 100.0 | | | |__ Oceanospirillales 73 72 98.6 | | | | |__ Oceanospirillaceae -

Life at Low Water Activity
Published online 12 July 2004 Life at low water activity W. D. Grant Department of Infection, Immunity and Inflammation, University of Leicester, Maurice Shock Building, University Road, Leicester LE1 9HN, UK ([email protected]) Two major types of environment provide habitats for the most xerophilic organisms known: foods pre- served by some form of dehydration or enhanced sugar levels, and hypersaline sites where water availability is limited by a high concentration of salts (usually NaCl). These environments are essentially microbial habitats, with high-sugar foods being dominated by xerophilic (sometimes called osmophilic) filamentous fungi and yeasts, some of which are capable of growth at a water activity (aw) of 0.61, the lowest aw value for growth recorded to date. By contrast, high-salt environments are almost exclusively populated by prokaryotes, notably the haloarchaea, capable of growing in saturated NaCl (aw 0.75). Different strategies are employed for combating the osmotic stress imposed by high levels of solutes in the environment. Eukaryotes and most prokaryotes synthesize or accumulate organic so-called ‘compatible solutes’ (osmolytes) that have counterbalancing osmotic potential. A restricted range of bacteria and the haloar- chaea counterbalance osmotic stress imposed by NaCl by accumulating equivalent amounts of KCl. Haloarchaea become entrapped and survive for long periods inside halite (NaCl) crystals. They are also found in ancient subterranean halite (NaCl) deposits, leading to speculation about survival over geological time periods. Keywords: xerophiles; halophiles; haloarchaea; hypersaline lakes; osmoadaptation; microbial longevity 1. INTRODUCTION aw = P/P0 = n1/n1 ϩ n2, There are two major types of environment in which water where n is moles of solvent (water); n is moles of solute; availability can become limiting for an organism. -

Genome Sequence and Description of Haloferax Massiliense Sp. Nov., a New Halophilic Archaeon Isolated from the Human Gut
Extremophiles (2018) 22:485–498 https://doi.org/10.1007/s00792-018-1011-1 ORIGINAL PAPER Genome sequence and description of Haloferax massiliense sp. nov., a new halophilic archaeon isolated from the human gut Saber Khelaifa1 · Aurelia Caputo1 · Claudia Andrieu1 · Frederique Cadoret1 · Nicholas Armstrong1 · Caroline Michelle1 · Jean‑Christophe Lagier1 · Felix Djossou2 · Pierre‑Edouard Fournier1 · Didier Raoult1,3 Received: 14 November 2017 / Accepted: 5 February 2018 / Published online: 12 February 2018 © The Author(s) 2018. This article is an open access publication Abstract By applying the culturomics concept and using culture conditions containing a high salt concentration, we herein isolated the frst known halophilic archaeon colonizing the human gut. Here we described its phenotypic and biochemical characteriza- tion as well as its genome annotation. Strain Arc-HrT (= CSUR P0974 = CECT 9307) was mesophile and grew optimally at 37 °C and pH 7. Strain Arc-HrT was also extremely halophilic with an optimal growth observed at 15% NaCl. It showed gram-negative cocci, was strictly aerobic, non-motile and non-spore-forming, and exhibited catalase and oxidase activities. The 4,015,175 bp long genome exhibits a G + C% content of 65.36% and contains 3911 protein-coding and 64 predicted RNA genes. PCR-amplifed 16S rRNA gene of strain Arc-HrT yielded a 99.2% sequence similarity with Haloferax prahovense, the phylogenetically closest validly published species in the Haloferax genus. The DDH was of 50.70 ± 5.2% with H. prahovense, 53.70 ± 2.69% with H. volcanii, 50.90 ± 2.64% with H. alexandrinus, 52.90 ± 2.67% with H. -

Haloferax Massiliensis Sp. Nov., the First Human-Associated Halophilic
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector NEW SPECIES Haloferax massiliensis sp. nov., the first human-associated halophilic archaea S. Khelaifia1,2 and D. Raoult1,2 1) Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, CNRS (UMR 7278), IRD (198), INSERM (U1095), AMU (UM63) and 2) Institut Hospitalo-Universitaire Méditerranée-Infection, Faculté de médecine, Aix-Marseille Université, Marseille, France Abstract We report the main characteristics of Haloferax massiliensis strain Arc-HrT (= CSUR P974) isolated from stool specimen of a 22-year-old Amazonian obese female patient. © 2016 The Authors. Published by Elsevier Ltd on behalf of European Society of Clinical Microbiology and Infectious Diseases. Keywords: Culturomics, genomics, Haloferax massiliensis, taxonogenomics, taxonomy Original Submission: 6 May 2016; Revised Submission: 9 May 2016; Accepted: 10 May 2016 Article published online: 14 May 2016 low speed. The pure culture of this halophilic archaea grew Corresponding author: S. Khelaifia, URMITE, CNRS (UMR 7278), aerobically after 7-day incubation at 37°C. Strain Arc-Hr ex- IRD (198), INSERM (U1095), AMU (UM63), Faculté de Médecine, Aix-Marseille Université, 27 Boulevard Jean Moulin, 13385 Marseille hibits positive catalase and oxidase activities. The growing col- Cedex 5, France onies on the halophilic medium were red, opaque and 0.5 to fi E-mail: khelai [email protected] 1 mm in diameter. Cells were Gram-negative cocci, nonmotile and non–spore forming with a diameter of 0.9 μm. The 16S rRNA gene was sequenced using the primers as previously described [4] using a 3130-XL sequencer (Applied Biosciences, In December 2013, we successfully isolated the strain Arc-Hr Saint Aubin, France). -

Pan-Genome Analysis and Ancestral State Reconstruction Of
www.nature.com/scientificreports OPEN Pan‑genome analysis and ancestral state reconstruction of class halobacteria: probability of a new super‑order Sonam Gaba1,2, Abha Kumari2, Marnix Medema 3 & Rajeev Kaushik1* Halobacteria, a class of Euryarchaeota are extremely halophilic archaea that can adapt to a wide range of salt concentration generally from 10% NaCl to saturated salt concentration of 32% NaCl. It consists of the orders: Halobacteriales, Haloferaciales and Natriabales. Pan‑genome analysis of class Halobacteria was done to explore the core (300) and variable components (Softcore: 998, Cloud:36531, Shell:11784). The core component revealed genes of replication, transcription, translation and repair, whereas the variable component had a major portion of environmental information processing. The pan‑gene matrix was mapped onto the core‑gene tree to fnd the ancestral (44.8%) and derived genes (55.1%) of the Last Common Ancestor of Halobacteria. A High percentage of derived genes along with presence of transformation and conjugation genes indicate the occurrence of horizontal gene transfer during the evolution of Halobacteria. A Core and pan‑gene tree were also constructed to infer a phylogeny which implicated on the new super‑order comprising of Natrialbales and Halobacteriales. Halobacteria1,2 is a class of phylum Euryarchaeota3 consisting of extremely halophilic archaea found till date and contains three orders namely Halobacteriales4,5 Haloferacales5 and Natrialbales5. Tese microorganisms are able to dwell at wide range of salt concentration generally from 10% NaCl to saturated salt concentration of 32% NaCl6. Halobacteria, as the name suggests were once considered a part of a domain "Bacteria" but with the discovery of the third domain "Archaea" by Carl Woese et al.7, it became part of Archaea.