VS005 Appendix a Declarable, Restricted and Prohibited Substances List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hidden Order Revealed Dilute Magnetic Semiconductors Such As Gallium Manganese Arsenide Could Be Key to the Development of Spintronics
news & views DILUTE MAGNETIC SEMICONDUCTORS Hidden order revealed Dilute magnetic semiconductors such as gallium manganese arsenide could be key to the development of spintronics. But the relationship between electronic transport and magnetic properties has been hotly debated. Data indicating the preservation of the non-magnetic character of the host material provide startling new insight. Michael E. Flatté magine striking a golf ball from a tee states may become high enough to form Ordinarily, electric current moves in the middle of a dense forest. !e ball extended-like states, contributing su&cient through ferromagnetic (Ga,Mn)As like a Iwould hit leaves, branches and trunks carriers to screen the disorder and allow golf ball that’s being continually hit through in rapid progression and not travel far. coherent charge transport similar to that a forest — slowly and incoherently. To But if the ball were hit from a tee on a associated with valence-band conduction. study the transport characteristics and platform suspended above the trees, it !e nature of the transport and electronic structure of the states in this would be free to "y considerably further. electronic structure in the prototypical material above the Fermi energy, Ohya A similar demonstration has now been dilute magnetic semiconductor (Ga,Mn) and co-workers1 constructed a series performed by Ohya et al. within the As has been the subject of lengthy debate. of resonant tunnelling diodes in which dilute magnetic semiconductor gallium At high levels of manganese doping, the carriers are injected from a non-magnetic manganese arsenide, (Ga,Mn)As, as material exhibits some extended-state semiconductor into a (Ga,Mn)As quantum reported in Nature Physics1. -

Activity 8 How Atoms Interact with Each Other
CS_Ch7_PeriodicTbl 4/27/06 1:45 PM Page 442 The Periodic Table Activity 8 How Atoms Interact with Each Other GOALS What Do You Think? In this activity you will: You have learned that the chemical behavior of an atom is • Relate patterns in ionization determined by the arrangement of the atom’s electrons, energies of elements to specifically the valence electrons. The salt that you put on patterns in electron your food is chemically referred to as NaCl—sodium chloride. arrangements. • Use your knowledge of • How might the valence electrons of sodium (Na) and electron arrangements and chlorine (Cl) interact to create this bond? valence electrons to predict formulas for compounds Record your ideas about this question in your Active formed by two elements. Chemistry log. Be prepared to discuss your responses with • Contrast ionic bonding and your small group and the class. covalent bonding. • Draw electron-dot diagrams Investigate for simple molecules with 1. In Activity 3 you read that John Dalton assumed that covalent bonding. chemical compounds formed from two elements combined in the simplest possible combination—one atom of each element. In Activity 6 you began to see that an atom’s chemical behavior reflects its excess or deficiency of electrons relative to an atom of the closest noble gas on the periodic table. Use the list of ionization energies in Activity 6 to answer the following questions: 442 Active Chemistry CS_Ch7_PeriodicTbl 2/28/05 10:04 AM Page 443 Activity 8 How Atoms Interact with Each Other a) Which atoms have the smallest stable electron arrangement as neon. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Effect of Mercury on Engineering Materials Used in Ammonia Plants
Effect of Mercury on Engineering Materials Used in Ammonia Plants Inappropriate combinations of moisture, alloy and mercury can accelerate corrosion and form explosive compounds. Are there ways of preventing these potential hazards? S.Mark Wilhelm Cortest Laboratories, Inc., Cypress, TX 77429 INTRODUCTION Secondly, concentration of mercury in LNG and ethylene plants has Elemental mercury (Hg) is becoming presented a range of materials an increasingly more prevalent related problems having to do with contaminant to natural gas feed aluminum. Lastly, reaction of stocks due to its production from mercury with ammonia (or NH3 various reservoirs around the world. precursors) can deposit potentially Table 1 shows the origin of gas explosive compounds in NH3 process having mercury contamination from a equipment. variety of locations(1>. This paper discusses the following In general, mercury contamination of points relating to mercury and its gas streams does not cause major influence on gas processing and use: operational problems for gas production, transportation or 1. Origin and composition of processing, at least from the mercury and mercury compounds standpoint of engineering. There in natural gas. are, however, environmental concerns from end use"'. Environmental issues Materials degradation will not be addressed in the present mechanisms. discussion. 3. Overview of mercury related Several situations have come to equipment failures. notice since 1975 that cause concern for the gas industry as they relate 4. Mercury - nitrogen compounds. to mercury contaminated gas. One such problem is that minuscule 5. Recommendations for ammonia amounts of mercury in gas can induce plant operations. liquid metal cracking of a small group of susceptible alloys. -

"Sony Mobile Critical Substance List" At
Sony Mobile Communications Public 1(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Prepared by Date SEM/CGQS JOHAN K HOLMQVIST 2014-02-20 Contents responsible if other than preparer Remarks This document is managed in metaDoc. Approved by SEM/CGQS (PER HÖKFELT) Sony Mobile Critical Substances Second Edition Contents 1 Revision history.............................................................................................3 2 Purpose.........................................................................................................3 3 Directive ........................................................................................................4 4 Application ....................................................................................................4 4.1 Terms and Definitions ...................................................................................4 5 Comply to Sony Technical Standard SS-00259............................................6 6 The Sony Mobile list of Critical substances in products................................6 7 The Sony Mobile list of Restricted substances in products...........................7 8 The Sony Mobile list of Monitored substances .............................................8 It is the responsibility of the user to ensure that they have a correct and valid version. Any outdated hard copy is invalid and must be removed from possible use. Public 2(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Substance control Sustainability is the backbone -
Printed Copies for Reference Only
Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 APPROVERS INFORMATION PREPARED BY: Jennilyn Rivera Dinglasan Title: EHS DATE: 5/3/2017 2:35:30 AM APPROVED BY: Jennilyn DATE: 8/10/2017 7:00:05 Rivera Dinglasan Title: EHS PM DATE: 7/21/2017 2:43:17 APPROVED BY: Aline Zeng Title: Purchasing AM APPROVED BY: Bill Hemrich Title: Purchasing DATE: 6/1/2017 3:28:35 PM DATE: 6/11/2017 11:05:48 APPROVED BY: Jade Yuan Title: Purchasing PM APPROVED BY: Matthew DATE: 5/26/2017 6:54:14 Briggs Title: Purchasing AM DATE: 5/26/2017 3:56:07 APPROVED BY: Michael Ji Title: Purchasing AM DATE: 8/31/2017 4:24:35 APPROVED BY: Olga Chen Title: Purchasing AM APPROVED BY: Alfredo DATE: 6/2/2017 11:54:20 Heredia Title: Supplier Quality AM APPROVED BY: Audrius DATE: 5/31/2017 1:20:01 Sutkus Title: Supplier Quality AM DATE: 5/26/2017 2:03:53 APPROVED BY: Sam Peng Title: Supplier Quality AM APPROVED BY: Arsenio DATE: 6/28/2017 2:43:49 Mabao Cesista Jr. Title: EHS Manager AM Printed copies for reference only Printed copies for reference only Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 1.0 Purpose and Scope 1.1 This specification provides general requirements to suppliers regarding Littelfuse Inc’s EHS specification with regards to regulatory compliance, EHS management systems, banned and restricted substances, packaging, and product environmental content reporting. 1.2 This specification applies to all equipment, materials, parts, components, packaging, or products supplied to Littelfuse, Inc. -

Ferromagnetic Compounds of Manganese with Group V-A Elements
FEBBOKAGIETIC COMPOUNDS OF MANGANESE WITH GROUP V-A ELEMENTS bf lEITH HOLCOMB SWEENY A THESIS aubll1tted '<> OREGON STATE COLLEGE in partial tult1llment ot the requirements tor the degree ot DOCTOR OF PHILOSOPHY June 1951 "rttnflmr Redacted for Privacy 5f*rlf }rofrrlor d {tr*rtrf I: *rrtt of lrrr Redacted for Privacy lHfilf of thr frerrttnt d &r!,rtrf Redacted for Privacy 0hrrrrrp of SlDeol Grnnrlr 0rll,ttro Redacted for Privacy Iu}.tr *dmtr E*eol ht &d.r 1r $p.A bf lirramr hrff ACDOWLEDGMEHTS The author wishes to express his gratitude to Dr. Allen B. Scott tor hie enthuaiaat1c and untiring interest in this 1nYest1gat1on; hie helpful guidance la a1ncerelJ appreciated. To those other members or the Chem1str7 and Physics departments who have aided ln this work, a grateful thank you. A large portion or this work was conducted while the author held a fellowship given b7 the E. I. du Pont de Nemours Comp&n7. The honor and aaaiatanoe given bJ tbia tellovahip is gratetull7 acknowledged. TABLE OF CONTENTS SUBJECT PAGE IKTRODUCTION 1 ObJects 1 B. FUNDAMENTAL RELATIONSHIPS OF FERROMAGIET IC THEORY 0 Ew1ng 1 s Contribution 6 fhe Weiss Thttory . 7 Bei~te:nberg Theory of Je!"romagnet1sm 14 Exchange Integral Oonfl1'11ons 16 C. PREPARA'l'IOli OF TSE SAMPLES 22 History 22 Allo1s Produced by Direct Comb1natt.on 26 Chemical. and Electrolytic Preparation ot Al1oJa 29 Crystallization Experiments 30 Tube Furnace Method ot Preparation ot Alloye33 Analya1a or Samples 39 Rhenium- Antimony Alloy 42 D, EXPERIMENTAL 45 Temperatare Meaauremen t 46 Thermoregulator 47 Dilatometer 52 Permeameter 57 Thermoelectric Potential 62 'fhermogal1'an1c Potential 65 Sugge·ated Improvements in the Apparatus 66 E . -

ROHS Annex II Dossier for Beryllium and Its Compounds. Restriction Proposal for Substances in Electrical and Electronic Equipment Under Rohs
www.oeko.de ROHS Annex II Dossier for Beryllium and its compounds. Restriction proposal for substances in electrical and electronic equipment under RoHS Report No. 5 Version 3 Substance Name: Beryllium and its compounds 25/03/2020 EC Numbers: Beryllium metal: 231-150-7 Beryllium oxide (BeO): 215-133-1 and other Beryllium compounds CAS Numbers: Beryllium metal: 7440-41-7 Beryllium oxide (BeO): 1304-56-9 and other beryllium compounds Head Office Freiburg P.O. Box 17 71 79017 Freiburg Street address Merzhauser Strasse 173 79100 Freiburg Tel. +49 761 45295-0 Office Berlin Schicklerstrasse 5-7 10179 Berlin Tel. +49 30 405085-0 Office Darmstadt Rheinstrasse 95 64295 Darmstadt Tel. +49 6151 8191-0 [email protected] www.oeko.de RoHS Annex II Dossier, Version 3 Beryllium and its compounds Table of Contents List of Figures 5 List of Tables 5 1 IDENTIFICATION, CLASSIFICATION AND LABELLING, LEGAL STATUS AND USE RESTRICTIONS 8 1.1 Identification 8 1.1.1 Name, other identifiers, and composition of the substance 9 1.1.2 Physico-chemical properties 10 1.2 Classification and labelling status 12 1.3 Legal status and use restrictions 14 1.3.1 Regulation of the substance under REACH 14 1.3.2 Occupational Exposure Limits (OEL) 14 1.3.3 Other legislative measures 15 1.3.4 Non-governmental and non-regulatory initiatives 15 2 USE IN ELECTRICAL AND ELECTRONIC EQUIPMENT 17 2.1 Function of the substance 17 2.2 Types of applications / types of materials 19 2.3 Quantities of the substance used 25 3 HUMAN HEALTH HAZARD PROFILE 29 3.1 Critical endpoint 29 3.2 Existing Guidance -

Sem Icond Uctors: Data Handbook
Otfried Madelung Sem icond uctors: Data Handbook 3rdedition Springer Short table of contents (for a more detailed table of contents see the following pages) A Introduction 1 General remarks to the structure of the volume .................................................................................... 1 2 Physical quantities tabulated in this volume ......................................................................................... 2 B Tetrahedrally bonded elements and compounds 1 Elements ofthe IVth group and IV-IV compounds ................................................ ....................... 7 2 111-V compounds .................................................................. 3 11-VI compounds ..................................................................................................... 4 I-VI1 compounds .................................................. ...................................................... 245 5 II12-V13 compounds .................................................................................................. 6 I-III-V12 compounds ..................................................................... 7 II-IV-V2 compounds ......................... ............................................................................... 329 8 I2-IV-VI3 compounds .......................................................................... ............................. 359 9 13-V-vI4 compounds ......................... ...................................................................................... 367 -

148 Subpart A—General Subpart B—Table of Hazardous Materials
§ 172.1 49 CFR Ch. I (10–1–15 Edition) 172.510 Special placarding provisions: Rail. APPENDIX B TO PART 172—TREFOIL SYMBOL 172.512 Freight containers and aircraft unit APPENDIX C TO PART 172—DIMENSIONAL SPEC- load devices. IFICATIONS FOR RECOMMENDED PLACARD 172.514 Bulk packagings. HOLDER 172.516 Visibility and display of placards. APPENDIX D TO PART 172—RAIL RISK ANAL- 172.519 General specifications for placards. YSIS FACTORS 172.521 DANGEROUS placard. AUTHORITY: 49 U.S.C. 5101–5128, 44701; 49 172.522 EXPLOSIVES 1.1, EXPLOSIVES 1.2 CFR 1.81, 1.96 and 1.97. and EXPLOSIVES 1.3 placards. 172.523 EXPLOSIVES 1.4 placard. SOURCE: Amdt. 172–29, 41 FR 15996, Apr. 15, 172.524 EXPLOSIVES 1.5 placard. 1976, unless otherwise noted. 172.525 EXPLOSIVES 1.6 placard. 172.526 [Reserved] 172.527 Background requirements for cer- Subpart A—General tain placards. 172.528 NON-FLAMMABLE GAS placard. § 172.1 Purpose and scope. 172.530 OXYGEN placard. This part lists and classifies those 172.532 FLAMMABLE GAS placard. materials which the Department has 172.536 [Reserved] designated as hazardous materials for 172.540 POISON GAS placard. purposes of transportation and pre- 172.542 FLAMMABLE placard. 172.544 COMBUSTIBLE placard. scribes the requirements for shipping 172.546 FLAMMABLE SOLID placard. papers, package marking, labeling, and 172.547 SPONTANEOUSLY COMBUSTIBLE transport vehicle placarding applicable placard. to the shipment and transportation of 172.548 DANGEROUS WHEN WET placard. those hazardous materials. 172.550 OXIDIZER placard. 172.552 ORGANIC PEROXIDE placard. [Amdt. 172–29, 41 FR 15997, Apr. -

Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005)
NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 IUPAC Periodic Table of the Elements 118 1 2 21314151617 H He 3 4 5 6 7 8 9 10 Li Be B C N O F Ne 11 12 13 14 15 16 17 18 3456 78910 11 12 Na Mg Al Si P S Cl Ar 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 55 56 * 57− 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba lanthanoids Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 87 88 ‡ 89− 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra actinoids Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo * 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ‡ 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr International Union of Pure and Applied Chemistry Nomenclature of Inorganic Chemistry IUPAC RECOMMENDATIONS 2005 Issued by the Division of Chemical Nomenclature and Structure Representation in collaboration with the Division of Inorganic Chemistry Prepared for publication by Neil G. -
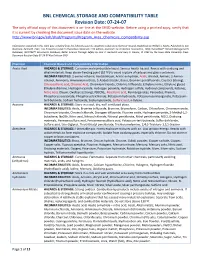
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers