Activity 8 How Atoms Interact with Each Other
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

"Sony Mobile Critical Substance List" At
Sony Mobile Communications Public 1(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Prepared by Date SEM/CGQS JOHAN K HOLMQVIST 2014-02-20 Contents responsible if other than preparer Remarks This document is managed in metaDoc. Approved by SEM/CGQS (PER HÖKFELT) Sony Mobile Critical Substances Second Edition Contents 1 Revision history.............................................................................................3 2 Purpose.........................................................................................................3 3 Directive ........................................................................................................4 4 Application ....................................................................................................4 4.1 Terms and Definitions ...................................................................................4 5 Comply to Sony Technical Standard SS-00259............................................6 6 The Sony Mobile list of Critical substances in products................................6 7 The Sony Mobile list of Restricted substances in products...........................7 8 The Sony Mobile list of Monitored substances .............................................8 It is the responsibility of the user to ensure that they have a correct and valid version. Any outdated hard copy is invalid and must be removed from possible use. Public 2(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Substance control Sustainability is the backbone -
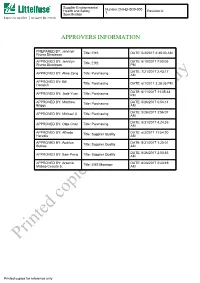
Printed Copies for Reference Only
Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 APPROVERS INFORMATION PREPARED BY: Jennilyn Rivera Dinglasan Title: EHS DATE: 5/3/2017 2:35:30 AM APPROVED BY: Jennilyn DATE: 8/10/2017 7:00:05 Rivera Dinglasan Title: EHS PM DATE: 7/21/2017 2:43:17 APPROVED BY: Aline Zeng Title: Purchasing AM APPROVED BY: Bill Hemrich Title: Purchasing DATE: 6/1/2017 3:28:35 PM DATE: 6/11/2017 11:05:48 APPROVED BY: Jade Yuan Title: Purchasing PM APPROVED BY: Matthew DATE: 5/26/2017 6:54:14 Briggs Title: Purchasing AM DATE: 5/26/2017 3:56:07 APPROVED BY: Michael Ji Title: Purchasing AM DATE: 8/31/2017 4:24:35 APPROVED BY: Olga Chen Title: Purchasing AM APPROVED BY: Alfredo DATE: 6/2/2017 11:54:20 Heredia Title: Supplier Quality AM APPROVED BY: Audrius DATE: 5/31/2017 1:20:01 Sutkus Title: Supplier Quality AM DATE: 5/26/2017 2:03:53 APPROVED BY: Sam Peng Title: Supplier Quality AM APPROVED BY: Arsenio DATE: 6/28/2017 2:43:49 Mabao Cesista Jr. Title: EHS Manager AM Printed copies for reference only Printed copies for reference only Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 1.0 Purpose and Scope 1.1 This specification provides general requirements to suppliers regarding Littelfuse Inc’s EHS specification with regards to regulatory compliance, EHS management systems, banned and restricted substances, packaging, and product environmental content reporting. 1.2 This specification applies to all equipment, materials, parts, components, packaging, or products supplied to Littelfuse, Inc. -

ROHS Annex II Dossier for Beryllium and Its Compounds. Restriction Proposal for Substances in Electrical and Electronic Equipment Under Rohs
www.oeko.de ROHS Annex II Dossier for Beryllium and its compounds. Restriction proposal for substances in electrical and electronic equipment under RoHS Report No. 5 Version 3 Substance Name: Beryllium and its compounds 25/03/2020 EC Numbers: Beryllium metal: 231-150-7 Beryllium oxide (BeO): 215-133-1 and other Beryllium compounds CAS Numbers: Beryllium metal: 7440-41-7 Beryllium oxide (BeO): 1304-56-9 and other beryllium compounds Head Office Freiburg P.O. Box 17 71 79017 Freiburg Street address Merzhauser Strasse 173 79100 Freiburg Tel. +49 761 45295-0 Office Berlin Schicklerstrasse 5-7 10179 Berlin Tel. +49 30 405085-0 Office Darmstadt Rheinstrasse 95 64295 Darmstadt Tel. +49 6151 8191-0 [email protected] www.oeko.de RoHS Annex II Dossier, Version 3 Beryllium and its compounds Table of Contents List of Figures 5 List of Tables 5 1 IDENTIFICATION, CLASSIFICATION AND LABELLING, LEGAL STATUS AND USE RESTRICTIONS 8 1.1 Identification 8 1.1.1 Name, other identifiers, and composition of the substance 9 1.1.2 Physico-chemical properties 10 1.2 Classification and labelling status 12 1.3 Legal status and use restrictions 14 1.3.1 Regulation of the substance under REACH 14 1.3.2 Occupational Exposure Limits (OEL) 14 1.3.3 Other legislative measures 15 1.3.4 Non-governmental and non-regulatory initiatives 15 2 USE IN ELECTRICAL AND ELECTRONIC EQUIPMENT 17 2.1 Function of the substance 17 2.2 Types of applications / types of materials 19 2.3 Quantities of the substance used 25 3 HUMAN HEALTH HAZARD PROFILE 29 3.1 Critical endpoint 29 3.2 Existing Guidance -

Sem Icond Uctors: Data Handbook
Otfried Madelung Sem icond uctors: Data Handbook 3rdedition Springer Short table of contents (for a more detailed table of contents see the following pages) A Introduction 1 General remarks to the structure of the volume .................................................................................... 1 2 Physical quantities tabulated in this volume ......................................................................................... 2 B Tetrahedrally bonded elements and compounds 1 Elements ofthe IVth group and IV-IV compounds ................................................ ....................... 7 2 111-V compounds .................................................................. 3 11-VI compounds ..................................................................................................... 4 I-VI1 compounds .................................................. ...................................................... 245 5 II12-V13 compounds .................................................................................................. 6 I-III-V12 compounds ..................................................................... 7 II-IV-V2 compounds ......................... ............................................................................... 329 8 I2-IV-VI3 compounds .......................................................................... ............................. 359 9 13-V-vI4 compounds ......................... ...................................................................................... 367 -

Technical Readiness and Gaps Analysis of Commercial Optical Materials and Measurement Systems for Advanced Small Modular Reactors
PNNL-22622, Rev. 1 SMR/ICHMI/PNNL/TR-2013/04 Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830 Technical Readiness and Gaps Analysis of Commercial Optical Materials and Measurement Systems for Advanced Small Modular Reactors NC Anheier M Bliss JD Suter BD Cannon A Qiao R Devanathan ES Andersen A Mendoza EJ Berglin DM Sheen August 2013 PNNL-22622, Rev. 1 SMR/ICHMI/PNNL/TR-2013/04 Technical Readiness and Gaps Analysis of Commercial Optical Materials and Measurement Systems for Advanced Small Modular Reactors NC Anheier M Bliss JD Suter BD Cannon A Qiao R Devanathan ES Andersen A Mendoza EJ Berglin DM Sheen August 2013 Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830 Pacific Northwest National Laboratory Richland, Washington 99352 Executive Summary This report supports the U.S. Department of Energy’s Office of Nuclear Energy (DOE-NE) Nuclear Energy Research and Development Roadmap and industry stakeholders by evaluating optically based instrumentation and control (I&C) concepts for advanced small modular reactor (AdvSMR) applications. These advanced designs will require innovative thinking in terms of engineering approaches, materials integration, and I&C concepts to realize their eventual viability and deployment. The primary goals of this report include: 1. Establish preliminary I&C needs, performance requirements, and possible gaps for AdvSMR designs based on best-available published design data. 2. Document commercial off-the-shelf (COTS) optical sensors, components, and materials in terms of their technical readiness to support essential AdvSMR in-vessel I&C systems. 3. Identify technology gaps by comparing the in-vessel monitoring requirements and environmental constraints to COTS optical sensor and materials performance specifications. -
Annex 1. Management Criteria for Substances of Environmental Concern (Automobile Products)
Annex 1. Management Criteria for Substances of Environmental Concern (Automobile Products) 1. Substances subject to management and management classifications We have adopted GADSL for our Management Criteria for Substances of Environmental Concern (Automobile Products). The management classifications and definitions for "prohibited substances" and "declarable substances" are shown in Table 1. [Precautions for use] The specific chemical substances within GADSL only enumerate some of the chemical substances that fall under their class of chemical substances. It does not regulate every chemical substance that falls under said class of chemical substances. The Japanese translation has been included for reference purposes, but there are some substances which do not have official Japanese names. The latest information on GADSL must be obtained and confirmed from the following website. [GADSL] The Global Automotive Declarable Substance List (GADSL) released by the Global Automotive Stakeholder Group (GASG) http://www.gadsl.org/ Table 1. Stanley's management classifications and definitions The reported substances contained within vehicle materials and parts have been indicated as either "P" or "D" via the following definitions. Classification Definition Target substances Response Substances that fall under Category P and those that fall under P of Category Promptly prohibit. Substances prohibited D/P of the substances of environmental from inclusion in the concern stipulated by GADSL Prohibited products, parts, materials, Four substances stipulated in the and secondary materials Prohibit all except for exempt parts European End of Life Vehicles (ELV) used at Stanley Electric stipulated in the European ELV Directive (lead, mercury, hexavalent Directive. chromium, and cadmium) Substances that must be reported to the company Substances that fall under Category D when they are used in the and those that fall under D of Category The supplier must promptly report to Declarable products, parts, materials, D/P of the substances of environmental Stanley. -

Management Standard for Banned & Controlled Substances Uap.01226 Ab
Proprietary Information of: Title: Document No. Rev. MANAGEMENT STANDARD FOR BANNED UAP.01226 AC & CONTROLLED SUBSTANCES For Reference Only When Printed Bez pečiatky len pre informáciu Solo per riferimento quando stampato 打印时仅供参考 Document revision verification may be obtained in Bel Fuse’s PLM system. Record of Revision: Rev Originator Description Approved By Date ECO AA M.Supakova Initial release E. Vergara 2017-04-20 C77008 AB M.Supakova Add limit for Halogen free product in table 1 E. Vergara 2019-10-31 C96739 AC M.Supakova Add TSCA, POPs requirements to table 1, E. Vergara 2021-08-27 CO111522 correct MCV Threshold according to customer specification, add point 6.5 regarding custom and industry specific requirements BPS Document Template UAF.01898 rev AC Page 1 of 112 Proprietary Information of: Title: Document No. Rev. MANAGEMENT STANDARD FOR BANNED UAP.01226 AC & CONTROLLED SUBSTANCES Contents 1 Purpose ...................................................................................................................................................................... 3 2 Scope.......................................................................................................................................................................... 3 3 Reference ................................................................................................................................................................... 3 4 Definition .................................................................................................................................................................... -

Iaea Tecdoc Series Tecdoc Iaea @
IAEA-TECDOC-1912 IAEA-TECDOC-1912 IAEA TECDOC SERIES Challenges for Coolants in Fast Neutron Spectrum Systems Challenges for Coolants in Fast IAEA-TECDOC-1912 Challenges for Coolants in Fast Neutron Spectrum Systems @ IAEA SAFETY STANDARDS AND RELATED PUBLICATIONS IAEA SAFETY STANDARDS Under the terms of Article III of its Statute, the IAEA is authorized to establish or adopt standards of safety for protection of health and minimization of danger to life and property, and to provide for the application of these standards. The publications by means of which the IAEA establishes standards are issued in the IAEA Safety Standards Series. This series covers nuclear safety, radiation safety, transport safety and waste safety. The publication categories in the series are Safety Fundamentals, Safety Requirements and Safety Guides. Information on the IAEA’s safety standards programme is available on the IAEA Internet site http://www-ns.iaea.org/standards/ The site provides the texts in English of published and draft safety standards. The texts of safety standards issued in Arabic, Chinese, French, Russian and Spanish, the IAEA Safety Glossary and a status report for safety standards under development are also available. For further information, please contact the IAEA at: Vienna International Centre, PO Box 100, 1400 Vienna, Austria. All users of IAEA safety standards are invited to inform the IAEA of experience in their use (e.g. as a basis for national regulations, for safety reviews and for training courses) for the purpose of ensuring that they continue to meet users’ needs. Information may be provided via the IAEA Internet site or by post, as above, or by email to Offi [email protected]. -

Prediction of Thermal Conductivity and Strategies for Heat Transport Reduction in Bismuth : an Ab Initio Study
Prediction of thermal conductivity and strategies for heat transport reduction in bismuth : an ab initio study . Maksim Markov To cite this version: Maksim Markov. Prediction of thermal conductivity and strategies for heat transport reduction in bismuth : an ab initio study .. Physics [physics]. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLX010. tel-01438827 HAL Id: tel-01438827 https://pastel.archives-ouvertes.fr/tel-01438827 Submitted on 18 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 NNT : 2016SACLX010 Thèse de doctorat de l'Université Paris-Saclay préparée à l'École Polytechnique École doctorale n◦573 INTERFACES Spécialité de doctorat : Physique par Maksim Markov Prédiction de la conductivité thermique et stratégie de réduction du transport de la chaleur dans le bismuth : étude ab initio. Thèse présentée et soutenue à l'École Polytechnique, le 11 Mars 2016. Composition du Jury : Dr. Sylvie Hébert Présidente du jury Laboratoire CRISMAT UMR6508 CNRS et ENSICAEN Prof. Alan J. H. McGaughey Rapporteur Université Carnegie Mellon Prof. Sebastian Volz Rapporteur École Centrale de Paris Prof. Antoine Georges Examinateur Centre de Physique Théorique, École Polytechnique Prof. Francesco Mauri Examinateur Université de Rome "La Sapienzà", IMPMC Université Paris VI, CNRS Dr. -

Semiconductors: Data Handbook H Springer-Verlag Berlin Heidelberg Gmbh Otfried Madelung
Semiconductors: Data Handbook H Springer-Verlag Berlin Heidelberg GmbH Otfried Madelung Semiconductors: Data Handbook 3rd edition 123 Proff. Dr. Ot frie d Madelung Am Kornacker 18 35041 Marburg Germany The 1st ed. was published in 2 volumes in the series “Data in Science and Technology”. The 2nd revised ed. was published under title “Semiconductors – Basic Data” . Additional material to this book can be downloaded from http://extras.springer.com ISBN 978-3-642-62332-5 ISBN 978-3-642-18865-7 (eBook) DOI 10.1007/978-3-642-18865-7 Cataloging-in-Publication Data appliedf fof r Bibliographic infformation published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in the Deutsche Nationalbibliograffie ; detailed bibliographic data is available in the Internet at <http://dnb.ddb.de>. This work is subject to copyright. All rights are reserved, whether the whole or part of tfhe material is concerned, speciffically the rights off trans lation, reprinting, reuse of if llustrations, recitation, broadcasting, reproduction on microfiff lm or in other ways, and storage in data banks. Duplication of tfhis publication or parts thereof is permitte d only under the provisions of tfhe German Copyright Law offSeptem ber 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag..Vio lations are liable for prosecution under German Copyright Lawaaw .w © Springer-Verlag Berlin Heidelberg 1991, 1992, 1996, and 2004 Originally published by Springer-Verlag Berlin Heidelberg New York in 2004 Softcover reprint of the hardcover 3rd edition 2004 http://www.springeronline.com The use of general descriptive names, registered names, trademarks,, etc. -
000090540 DI Stoffliste-Restricted Substances List-Unterschrift
QES Document Stoffliste - Doc No 000090540 AVL LIST GmbH Liste deklarationspflichtiger und verbotener Core process: - Stoffe Revision: 06 Restricted Substances List Gültig ab: 12/2015 Änderungsprotokoll / Revision Protocol Revisionsdatum / Revision Änderungsgrund / Reason of Changes Revision Date 00 01/05/2002 Erstausgabe / first edition 01 15/10/2004 Geänderte Anforderungen / Altered requirements (Ford, Bosch and Volvo) 02 01/02/2008 Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault) 03 10/09/2008 Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault, GADSL) Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault, GADSL, 04 09/03/2009 Scania) 05 20/04/2012 Geänderte Anforderungen / Altered requirements (Bosch, Volvo) Geänderte Anforderungen / Altered requirements (Bosch, Ford, Kubota, Scania, 06 26/11/2015 Volvo) Name Datum / Date Unterschrift / Signature Erstellt / Created O. Herschmann 26/11/2015 Geprüft / Reviewed H. Reitbauer 26/11/2015 Freigegeben / Released O. Herschmann 26/11/2015 Public Copyright © 2015 AVL LIST GMBH, all rights reserved. 000090540_DI_Stoffliste-Restricted Substances List; R06 Uncontrolled print out, controlled version in Intranet. Page 1 of 47 Dok-Nr. 000090540 QES Dokument Kernprozess: - AVL LIST GmbH Stofflisten Revision: 06 Liste deklarationspflichtiger und verbotener Stoffe Gültig ab: 12/2015 Zweck: Dieses QES-Dokument dient der Definition von Stoffen, die nur eingeschränkt oder unter gewissen Rahmenbedingungen in der AVL List GmbH verwendet oder durch sie in Umlauf gebracht werden dürfen. Diese Stoffliste ergänzt die Verantwortung jedes Lieferanten, geltende, möglicherweise schärfere gesetzliche Vorschriften in der jeweils aktuellen Fassung einzuhalten. Geltungsbereich: Dieses QES-Dokument ist für alle Stoffe, Zubereitungen und Produkte inklusive deren Verpackungen anzuwenden, die an AVL geliefert, innerhalb AVL hergestellt, verwendet oder von AVL geliefert werden.