ASMP005 Appendix 2 : List of Substances (Note1)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Picrotoxin-Like Channel Blockers of GABAA Receptors
COMMENTARY Picrotoxin-like channel blockers of GABAA receptors Richard W. Olsen* Department of Molecular and Medical Pharmacology, Geffen School of Medicine, University of California, Los Angeles, CA 90095-1735 icrotoxin (PTX) is the prototypic vous system. Instead of an acetylcholine antagonist of GABAA receptors (ACh) target, the cage convulsants are (GABARs), the primary media- noncompetitive GABAR antagonists act- tors of inhibitory neurotransmis- ing at the PTX site: they inhibit GABAR Psion (rapid and tonic) in the nervous currents and synapses in mammalian neu- system. Picrotoxinin (Fig. 1A), the active rons and inhibit [3H]dihydropicrotoxinin ingredient in this plant convulsant, struc- binding to GABAR sites in brain mem- turally does not resemble GABA, a sim- branes (7, 9). A potent example, t-butyl ple, small amino acid, but it is a polycylic bicyclophosphorothionate, is a major re- compound with no nitrogen atom. The search tool used to assay GABARs by compound somehow prevents ion flow radio-ligand binding (10). through the chloride channel activated by This drug target appears to be the site GABA in the GABAR, a member of the of action of the experimental convulsant cys-loop, ligand-gated ion channel super- pentylenetetrazol (1, 4) and numerous family. Unlike the competitive GABAR polychlorinated hydrocarbon insecticides, antagonist bicuculline, PTX is clearly a including dieldrin, lindane, and fipronil, noncompetitive antagonist (NCA), acting compounds that have been applied in not at the GABA recognition site but per- huge amounts to the environment with haps within the ion channel. Thus PTX major agricultural economic impact (2). ͞ appears to be an excellent example of al- Some of the other potent toxicants insec- losteric modulation, which is extremely ticides were also radiolabeled and used to important in protein function in general characterize receptor action, allowing and especially for GABAR (1). -

1,1,1,2-Tetrafluoroethane
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 11 1,1,1,2-Tetrafluoroethane First draft prepared by Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United Kingdom Please not that the layout and pagination of this pdf file are not identical to the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization -

United States Patent (10) Patent No.: US 7,190,052 B2 Lindgren (45) Date of Patent: Mar
US0071900.52B2 (12) United States Patent (10) Patent No.: US 7,190,052 B2 Lindgren (45) Date of Patent: Mar. 13, 2007 (54) SEMICONDUCTOR DEVICES WITH OXIDE 5,516,721 A 5/1996 Galli et al. COATINGS SELECTIVELY POSITONED 5,683,946 A 1 1/1997 Lu et al. OVER EXPOSED FEATURES INCLUDING 5,776,829 A 7/1998 Homma et al. SEMCONDUCTOR MATERAL 6,022,814 A 2/2000 Mikoshiba et al. 6,045,877 A 4/2000 Gleason et al. (75) Inventor: Joseph T. Lindgren, Boise, ID (US) 6,174,8246,130,116 AB1 10/20001/2001 MichaelSmith et etal. al. 6, 197,110 B1 3/2001 Lee et al. (73) Assignee: Micron Technology, Inc., Boise, ID 6,251,753 B1 6/2001 Yeh et al. (US) (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 0 days. Schreiber, S.J. et al., “Low Temperature Deposition of Microcrystal line Silicon For Thin Film Transistors,' CUED Electronic Devices (21) Appl. No.: 10/454.256 and Materials Group, http://www2.eng.cam.ac.uk/~www-edm/ (22) Filed: Jun. 3, 2003 projects/lowtempdepf1.htm, prior to Sep. 2000, 2 pages. e as (Continued) (65) Prior Publication Data Primary Examiner T. N. Quach US 2004/OO33682 A1 Feb. 19, 2004 (74) Attorney, Agent, or Firm TraskBritt Related U.S. Application Data (57) ABSTRACT (62) Division of application No. 10/218.268, filed on Aug. 13, 2002, now Pat. No. 6,593,221. A semiconductor device structure includes a passivation layer through which only non-silicon-comprising structures (51) Int. -

Current Awareness in Clinical Toxicology Editors: Damian Ballam Msc and Allister Vale MD
Current Awareness in Clinical Toxicology Editors: Damian Ballam MSc and Allister Vale MD August 2016 CONTENTS General Toxicology 6 Metals 30 Management 14 Pesticides 31 Drugs 16 Chemical Warfare 33 Chemical Incidents & 24 Plants 34 Pollution Chemicals 25 Animals 34 CURRENT AWARENESS PAPERS OF THE MONTH Toxicity evaluation of α-pyrrolidinovalerophenone (α-PVP): results from intoxication cases within the STRIDA project Beck O, Franzén L, Bäckberg M, Signell P, Helander A. Clin Toxicol 2016; 54: 568-75. Context An increasing number of new psychoactive substances (NPS) of different chemical classes have become available through marketing and sale over the Internet. This report from the Swedish STRIDA project presents the prevalence, laboratory results, and clinical features in a series of intoxications involving the stimulant NPS -pyrrolidinovalerophenone (-PVP), a potent dopamine re-uptake inhibitor, over a 4-year period. Study design Observational case series of consecutive patients with admitted or suspected intake of NPS presenting to hospitals in Sweden from 2012 to 2015. Patients and methods In the STRIDA project, blood and urine samples are collected from intoxicated patients with admitted or suspected intake of NPS or unknown drugs presenting to hospitals over the country. Analysis of NPS is performed by mass spectrometry multicomponent methods. Clinical data are collected when caregivers consult the Swedish Poisons Information Centre Current Awareness in Clinical Toxicology is produced monthly for the American Academy of Clinical Toxicology by the Birmingham Unit of the UK National Poisons Information Service, with contributions from the Cardiff, Edinburgh, and Newcastle Units. The NPIS is commissioned by Public Health England 2 (PIC), and retrieved from medical records. -

SAFETY DATA SHEET Difluoromethane (R32) SECTION 1
SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 1/14 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Difluoromethane (R32) Other Name: HFC-32 Additional identification Chemical name: Difluoromethane Chemical formula: CH2F2 INDEX No. - CAS-No. 75-10-5 EC No. 200-839-4 REACH Registration No. 01-2119471312-47 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Refrigerant. Use as an Intermediate (transported, on-site isolated). Use for electronic component manufacture. Using gas alone or in mixtures for the calibration of analysis equipment. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 as amended. Physical Hazards Flammable gas Category 1 H220: Extremely flammable gas. Gases under pressure Liquefied gas H280: Contains gas under pressure; may explode if heated. SDS_AT - 000010021734 SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 2/14 2.2 Label Elements Signal Words: Danger Hazard Statement(s): H220: Extremely flammable gas. -

Hydrofluoric Acid Reduction Project – TURI Grant 2017
Hydrofluoric Acid Reduction Project – TURI Grant 2017 Sponsored by: Toxics Use Reduction Institute 126 John St Lowell, MA 01854 TURI Report 2018-001 Authors: Tyler DeFosse David Demarey Supervisor: Paul Watson Special Thanks to: Joy Onasch – Toxics Use Reduction Institute John Raschko – Massachusetts Office of Technical Assistance and Technology 1 Table of Contents 1.0 Executive Summary .......................................................................................................................... 3 2.0 Background ....................................................................................................................................... 5 2.1 Introduction ......................................................................................................................................... 5 2.2 Literature Searches .......................................................................................................................... 6 2.2.1 Diffusion Dialysis ...................................................................................................................... 6 2.2.2 Reverse Osmosis ....................................................................................................................... 7 2.2.3 Hydrofluoric Acid Wastewater Recycling Method ............................................................... 7 2.2.4 Issues Regarding the Etching of Silicon Dioxide ................................................................. 8 2.3 Alternative Strategies ..................................................................................................................... -

XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: http://www.tandfonline.com/loi/ictx20 XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta To cite this article: (2015) XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta, Clinical Toxicology, 53:4, 233-403, DOI: 10.3109/15563650.2015.1024953 To link to this article: http://dx.doi.org/10.3109/15563650.2015.1024953 Published online: 26 Mar 2015. Submit your article to this journal Article views: 3422 View related articles View Crossmark data Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ictx20 Download by: [UPSTATE Medical University Health Sciences Library] Date: 28 December 2016, At: 10:31 Clinical Toxicology (2015), 53, 233–403 Copyright © 2015 Informa Healthcare USA, Inc. ISSN: 1556-3650 print / 1556-9519 online DOI: 10.3109/15563650.2015.1024953 ABSTRACTS XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian ’ s, Malta 1. Modelling dose-concentration-response Introduction: The American Association of Poison Control Cen- ters (AAPCC) published its fi rst annual report in 1983. Call data Ursula Gundert-Remy from sixteen US poison centers was chronicled in that report. Seven submitted data for the entire year. By July 2000, 63 centers Institute for Clinical Pharmacology and Toxicology, Charit é were part of the national poison center system, but only 59 submit- Medical School, Berlin, Germany ted data for the full year. -

Activity 8 How Atoms Interact with Each Other
CS_Ch7_PeriodicTbl 4/27/06 1:45 PM Page 442 The Periodic Table Activity 8 How Atoms Interact with Each Other GOALS What Do You Think? In this activity you will: You have learned that the chemical behavior of an atom is • Relate patterns in ionization determined by the arrangement of the atom’s electrons, energies of elements to specifically the valence electrons. The salt that you put on patterns in electron your food is chemically referred to as NaCl—sodium chloride. arrangements. • Use your knowledge of • How might the valence electrons of sodium (Na) and electron arrangements and chlorine (Cl) interact to create this bond? valence electrons to predict formulas for compounds Record your ideas about this question in your Active formed by two elements. Chemistry log. Be prepared to discuss your responses with • Contrast ionic bonding and your small group and the class. covalent bonding. • Draw electron-dot diagrams Investigate for simple molecules with 1. In Activity 3 you read that John Dalton assumed that covalent bonding. chemical compounds formed from two elements combined in the simplest possible combination—one atom of each element. In Activity 6 you began to see that an atom’s chemical behavior reflects its excess or deficiency of electrons relative to an atom of the closest noble gas on the periodic table. Use the list of ionization energies in Activity 6 to answer the following questions: 442 Active Chemistry CS_Ch7_PeriodicTbl 2/28/05 10:04 AM Page 443 Activity 8 How Atoms Interact with Each Other a) Which atoms have the smallest stable electron arrangement as neon. -

Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Attachment 3-1 Guidance for Developing Ecological Soil
Attachment 3-1 Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs) Eco-SSL Standard Operating Procedure (SOP # 1): Plant and Soil Invertebrate Literature Search and Acquisition OSWER Directive 92857-55 November 2003 This page intentionally left blank OVERVIEW Currently, there is a lack of clear guidance in setting terrestrial effect thresholds when conducting risk assessments. Without an EPA-approved, peer-reviewed, ecologically-based terrestrial effect database, the process to develop thresholds is problematic both to EPA, other federal agencies, states, and concerned private parties. Identification of published toxicity studies on invertebrates, microbial processes and plants is a key step in the derivation of benchmarks. The purpose of the Task Group 4, Standard Operating Procedure Number 1: Literature Search and Acquisition (referred to as TG4-SOP#1) is to document procedures used to identify and acquire potentially relevant toxicology literature for use in setting ecological soil screening levels. The literature search strategy is designed to locate worldwide terrestrial toxicity literature that includes the effects of chemicals of concern on terrestrial soil-dwelling invertebrates and plants. The literature acquisition process is designed to ensure timely acquisition of relevant publications. LITERATURE IDENTIFICATION Potentially relevant literature for developing ecological soil screening levels (Eco-SSLs) is identified by examining hard copies of relevant journals, bibliographies and guidance publications and through the use of a comprehensive computerized literature search strategy. These procedures are designed to locate worldwide terrestrial toxicology literature that includes the effects of specific toxic substances with an emphasis on exposure via soil. Paper-based Literature Identification The paper-based literature identification process includes the scanning of relevant review article bibliographies and key journals held in the U.S. -
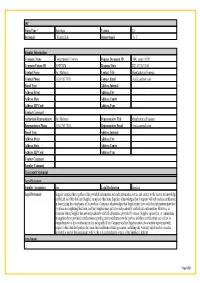
Material Composition Declaration
IPC Form Type * Distribute Version 2.0 Sectionals - MaterialInfo Subsectionals - A- C Supplier Information Company Name Contemporary Controls Request Document ID EISK_series_MCD Company Unique ID 363857474 Response Date 2021-07-30-12:00 Contact Name Neil Maloney Contact Title Manufacturing Engineer Contact Phone (630) 963 7070; Contact Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Authorized Representative Neil Maloney Representative Title Manufacturing Engineer Representative Phone (630) 963 7070; Representative Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Supplier Comment Uncertainty Statement Legal Statement Supplier Acceptance true Legal Declaration Standard Legal Statement Supplier certifies that it gathered the provided information and such information is true and correct to the best of its knowledge and belief, as of the date that Supplier completes this form. Supplier acknowledges that Company will rely on this certification in determining the compliance of its products. Company acknowledges that Supplier may have relied on information provided by others in completing this form, and that Supplier may not have independently verified such information. However, in situations where Supplier has not independently verified information provided by others, Supplier agrees that, at a minimum, its suppliers have provided certifications regarding their contributions to the part(s), and those certifications are at least as comprehensive as the certification in this paragraph. If the Company and the Supplier enter into a written agreement with respect to the identified part(s), the terms and conditions of that agreement, including any warranty rights and/or remedies provided as part of that agreement, will be the sole and exclusive source of the Supplier’s liability Attachment . -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium