Ferromagnetic Compounds of Manganese with Group V-A Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hidden Order Revealed Dilute Magnetic Semiconductors Such As Gallium Manganese Arsenide Could Be Key to the Development of Spintronics
news & views DILUTE MAGNETIC SEMICONDUCTORS Hidden order revealed Dilute magnetic semiconductors such as gallium manganese arsenide could be key to the development of spintronics. But the relationship between electronic transport and magnetic properties has been hotly debated. Data indicating the preservation of the non-magnetic character of the host material provide startling new insight. Michael E. Flatté magine striking a golf ball from a tee states may become high enough to form Ordinarily, electric current moves in the middle of a dense forest. !e ball extended-like states, contributing su&cient through ferromagnetic (Ga,Mn)As like a Iwould hit leaves, branches and trunks carriers to screen the disorder and allow golf ball that’s being continually hit through in rapid progression and not travel far. coherent charge transport similar to that a forest — slowly and incoherently. To But if the ball were hit from a tee on a associated with valence-band conduction. study the transport characteristics and platform suspended above the trees, it !e nature of the transport and electronic structure of the states in this would be free to "y considerably further. electronic structure in the prototypical material above the Fermi energy, Ohya A similar demonstration has now been dilute magnetic semiconductor (Ga,Mn) and co-workers1 constructed a series performed by Ohya et al. within the As has been the subject of lengthy debate. of resonant tunnelling diodes in which dilute magnetic semiconductor gallium At high levels of manganese doping, the carriers are injected from a non-magnetic manganese arsenide, (Ga,Mn)As, as material exhibits some extended-state semiconductor into a (Ga,Mn)As quantum reported in Nature Physics1. -

Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005)
NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 IUPAC Periodic Table of the Elements 118 1 2 21314151617 H He 3 4 5 6 7 8 9 10 Li Be B C N O F Ne 11 12 13 14 15 16 17 18 3456 78910 11 12 Na Mg Al Si P S Cl Ar 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 55 56 * 57− 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba lanthanoids Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 87 88 ‡ 89− 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra actinoids Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo * 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ‡ 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr International Union of Pure and Applied Chemistry Nomenclature of Inorganic Chemistry IUPAC RECOMMENDATIONS 2005 Issued by the Division of Chemical Nomenclature and Structure Representation in collaboration with the Division of Inorganic Chemistry Prepared for publication by Neil G. -

List of Semiconductor Materials - Wikipedia, the Free Encyclopedia Page 1 of 4
List of semiconductor materials - Wikipedia, the free encyclopedia Page 1 of 4 List of semiconductor materials From Wikipedia, the free encyclopedia Semiconductor materials are insulators at absolute zero temperature that conduct electricity in a limited way at room temperature. The defining property of a semiconductor material is that it can be doped with impurities that alter its electronic properties in a controllable way. Because of their application in devices like transistors (and therefore computers) and lasers, the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. The most commonly used semiconductor materials are crystalline inorganic solids. These materials can be classified according to the periodic table groups from which their constituent atoms come. Semiconductor materials are differing by their properties. Compound semiconductors have advantages and disadvantages in comparison with silicon. For example gallium arsenide has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows operation of power devices at higher temperatures, and gives lower thermal noise to low power devices at room temperature; its direct band gap gives it more favorable optoelectronic properties than the indirect band gap of silicon; it can be alloyed to ternary and quaternary compositions, with adjustable band gap width, allowing light emission at chosen wavelengths, and allowing e.g. matching to wavelengths with lowest losses in optical fibers. GaAs can be also grown in a semiinsulating form, which is suitable as a lattice-matching insulating substrate for GaAs devices. Conversely, silicon is robust, cheap, and easy to process, while GaAs is brittle, expensive, and insulation layers can not be created by just growing an oxide layer; GaAs is therefore used only where silicon is not sufficient.[1] Some materials can be prepared with tunable properties, e.g. -

Researchers Resolve Controversy Over Gallium Manganese Arsenide That Could Boost Spintronic Performance 27 February 2012, by Lynn Yarris
Researchers resolve controversy over gallium manganese arsenide that could boost spintronic performance 27 February 2012, by Lynn Yarris Lab's Materials Sciences Division and Margaret Dobrowolska, a physicist at Notre Dame, led a study in which it was shown that the holes (positively-charged energy spaces) in gallium manganese arsenide that control the Curie temperature, the temperature at which magnetism is lost, are located in an impurity energy band rather than a valence energy band, as many scientists have argued. This finding opens the possibility of fabricating gallium manganese arsenide so as to expand the width and occupation of the impurity band and thereby boost the Curie temperature to improve spintronic potential. "Our results challenge the valence band picture for gallium manganese arsenide and point to the existence of an impurity band, created by even Researchers with Berkeley Lab and Notre Dame moderate to high doping levels of manganese," University found that the spintronic properties of gallium manganese arsenide arise from holes in an impurity Walukiewicz says. "It is the location and partially band, created by manganese doping, that depletes the localized nature of holes within this impurity band valence band and shifts the Fermi level. Credit: Image that drives the value of the Curie temperature." courtesy of Walukiewicz, Dobrowolska, et. al. The results of this study have been published in the journal Nature Materials in a paper titled "Controlling the Curie temperature in (Ga,Mn)As A long-standing controversy regarding the through location of the Fermi level within the semiconductor gallium manganese arsenide, one impurity band." Co-authoring the paper with of the most promising materials for spintronic Walukiewicz and Dobrowolska were Kritsanu technology, looks to have been resolved. -

Novel Energy-Band Model for Semiconducting Spintronics Material Gallium Manganese Arsenide 29 March 2011
Novel energy-band model for semiconducting spintronics material gallium manganese arsenide 29 March 2011 (PhysOrg.com) -- The Fermi level position and Furthermore, the exchange splitting of the VB is band structure of gallium manganese arsenide has found to be very small (only several milli-eV), even been determined for the first time, shedding light in GaMnAs with a high Curie temperature (154K). on the precise mechanism behind ferromagnetism in the material. Researchers suggest that their findings shed light on the precise mechanism behind ferromagnetism The University of Tokyo announced on February 7, in GaMnAs; a subject that has been debated for 2011 that Professor M. Tanaka and his colleagues more than a decade. have succeeded in determining the Fermi level position and band structure in semiconducting More information: Shinobu Ohya, Kenta Takata spintronics material gallium manganese arsenide & Masaaki Tanaka, "Nearly non-magnetic valence (GaMnAs) by a unique method combining a band of the ferromagnetic semiconductor precise etching technique and resonant tunneling GaMnAs", Nature Physics (2011) spectroscopy. Details were presented in Nature DOI:10.1038/nphys1905 . Published online 06 Physics. February 2011. A ferromagnetic semiconductor is a basic material for spintronics which utilizes "electron spin" to realize new functional devices. Although GaMnAs Provided by National Institute for Materials is such a typical ferromagnetic material, its band Science structure has been controversial. Prevalent model assumes a valence band merged with an impurity band due to Mn atoms, with the Fermi level located inside the band and holes contribute to electrical conduction. However, optical study has shown that the Fermi level is located outside of valence band. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0240821 A1 CARROLL Et Al
US 20160240821A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0240821 A1 CARROLL et al. (43) Pub. Date: Aug. 18, 2016 (54) FREQUENCY DEPENDENT LIGHT HOIL 33/42 (2006.01) EMITTING DEVICES HOIL 33/30 (2006.01) (71) Applicant: WAKE FOREST UNIVERSITY, HOIL 33/00 (2006.01) Winston-Salem, NC (US) (52) U.S. Cl. CPC ........ HOIL 51/5296 (2013.01); HOIL 51/5206 (72) Inventors: David CARROLL, Winston-Salem, NC (2013.01); HOIL 51/5234 (2013.01); HOIL (US); Robert SUMMERS, Clemmons, 33/30 (2013.01); HOIL 33/004I (2013.01); NC(US); Yonghua CHEN, Cleveland HOIL 33/42 (2013.01); H05B33/08 (2013.01); Height, OH (US) HOIL 225 1/303 (2013.01); HOIL 5 1/5016 (21) Appl. No.: 15/024,624 (2013.01) (22) PCT Filed: Sep. 26, 2014 (57) ABSTRACT (86). PCT No.: PCT/US2014/057774 S371 (c)(1), (2) Date: Mar. 24, 2016 An electroluminescent device described herein, in one aspect, comprises a first electrode and second electrode and a light Related U.S. Application Data emitting layer positioned between the first and second elec trodes. A current injection gate is positioned between the first (60) Provisional application No. 61/883,710, filed on Sep. electrode and the light emitting layer or the second electrode 27, 2013. and the light emitting layer. In some embodiments, the cur Publication Classification rent injection gate comprises a semiconductor layer of elec tronic structure restricting injected current flow from the first (51) Int. Cl. or second electrode through the semiconductor layer as a HOIL 5/52 (2006.01) function of alternating current Voltage frequency applied to H05B33/08 (2006.01) the first and second electrodes. -
000090540 DI Stoffliste-Restricted Substances List-Unterschrift
QES Document Stoffliste - Doc No 000090540 AVL LIST GmbH Liste deklarationspflichtiger und verbotener Core process: - Stoffe Revision: 06 Restricted Substances List Gültig ab: 12/2015 Änderungsprotokoll / Revision Protocol Revisionsdatum / Revision Änderungsgrund / Reason of Changes Revision Date 00 01/05/2002 Erstausgabe / first edition 01 15/10/2004 Geänderte Anforderungen / Altered requirements (Ford, Bosch and Volvo) 02 01/02/2008 Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault) 03 10/09/2008 Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault, GADSL) Geänderte Anforderungen / Altered requirements (Ford, Bosch, Volvo, Renault, GADSL, 04 09/03/2009 Scania) 05 20/04/2012 Geänderte Anforderungen / Altered requirements (Bosch, Volvo) Geänderte Anforderungen / Altered requirements (Bosch, Ford, Kubota, Scania, 06 26/11/2015 Volvo) Name Datum / Date Unterschrift / Signature Erstellt / Created O. Herschmann 26/11/2015 Geprüft / Reviewed H. Reitbauer 26/11/2015 Freigegeben / Released O. Herschmann 26/11/2015 Public Copyright © 2015 AVL LIST GMBH, all rights reserved. 000090540_DI_Stoffliste-Restricted Substances List; R06 Uncontrolled print out, controlled version in Intranet. Page 1 of 47 Dok-Nr. 000090540 QES Dokument Kernprozess: - AVL LIST GmbH Stofflisten Revision: 06 Liste deklarationspflichtiger und verbotener Stoffe Gültig ab: 12/2015 Zweck: Dieses QES-Dokument dient der Definition von Stoffen, die nur eingeschränkt oder unter gewissen Rahmenbedingungen in der AVL List GmbH verwendet oder durch sie in Umlauf gebracht werden dürfen. Diese Stoffliste ergänzt die Verantwortung jedes Lieferanten, geltende, möglicherweise schärfere gesetzliche Vorschriften in der jeweils aktuellen Fassung einzuhalten. Geltungsbereich: Dieses QES-Dokument ist für alle Stoffe, Zubereitungen und Produkte inklusive deren Verpackungen anzuwenden, die an AVL geliefert, innerhalb AVL hergestellt, verwendet oder von AVL geliefert werden. -

United States Patent (19) 11 Patent Number: 5,998,528 Tsipursky Et Al
USOO5998528A United States Patent (19) 11 Patent Number: 5,998,528 Tsipursky et al. (45) Date of Patent: *Dec. 7, 1999 54 VISCOUS CARRIER COMPOSITIONS, 4,842,651 6/1989 Ravet et al. ............................ 106/487 INCLUDING GELS, FORMED WITH AN ORGANIC LIQUID CARRIER, A LAYERED (List continued on next page.) MATERIAL: POLYMER COMPLEX, AND A DI-, AND/OR TRI-VALENT CATION FOREIGN PATENT DOCUMENTS 0 205 281 A3 12/1986 European Pat. Off.. (75) Inventors: Semeon Tsipursky, Lincolnwood; 0 335 653 A1 10/1989 European Pat. Off.. Vladimir Dolinko, Libertyville; 0.358 415 A1 3/1990 European Pat. Off.. Vasiliki Psihogios, Elk Grove Village; 0 479 031A1 4/1992 European Pat. Off.. Gary W. Beall, McHenry, all of Ill. 0548940 A1 6/1993 European Pat. Off.. 0 761 739 A1 3/1997 European Pat. Off.. 1642 122 7/1970 Germany. 73) Assignee: Amcol International Corporation, 1 146 668 3/1969 United Kingdom. Arlington Heights, Ill. 1565 362 4/1980 United Kingdom. O 645 181 A2 3/1995 United Kingdom. Notice: This patent is subject to a terminal dis WO 93/04117 3/1993 WIPO. claimer. WO 93/04118 3/1993 WIPO. WO 93/11190 6/1993 WIPO. Appl. No.: 09/017,421 OTHER PUBLICATIONS Filed: Feb. 2, 1998 C. W. Francis, “Adsorption of Polyvinylpyrrolidone on Related U.S. Application Data Reference Clay Minerals”, Soil Science, vol. 115, No. 1, 1973, pp. 40–54. Continuation-in-part of application No. 08/525,416, Sep. 8, 1995, Pat. No. 5,721.306, and application No. 08/637,092, A. Usuki, et al., “Synthesis of nylon 6-clay hybrid”, J. -

Green References01 2018.Pdf
Toyota Boshoku Controled Substances List Updated: 2018/3/7 ※If you need to know exemption from banning of the use, please check "List of Exemption" sheet Japanese Environment regulation Japanese Safety regulation method of PRTR method of method of Poisonous and (specially 100 kind of VOC Deleterious TB Controled TB Banned PRTR PRTR Industrial Safety Ordinance on Prevention CAS№ Substance Name substances which Ordinance on Substances Substances Substances designated (Class (Class and Health Act. of Dangers due to Heavy New addition need to be notified Prevention of Management Substances subject Specified Chemical Carcinogen note for Class 1) 1) 2) by Japanese Organic Solvent Law. metals [Year/Month] to "GHS Substances. Japanese Environment Poisoning. Poisonous Japanese PRTR Japanese PRTR labeling"and"SDS Specified Chemical PRTR law Ministry Organic solvent Substances/Dele law law reception" Substances [specified chemical [Class 1] [Class 2] terious substances of Substances Category A ] specially 50-00-0 Formaldehyde ○ - 411 411 - 99 548 designated for - - ○ - Class 2 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5- 50-06-6 ○ - - - - - - - - - - ○ - phenyl- Azirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione, 6- amino-8-[[(aminocarbonyl)oxy]methyl]- 50-07-7 ○ - - - - - - - - - - ○ - 1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, [1aS-(1aa,8b,8aa,8ba)]- 50-18-0 Cyclophosphamide ○ - - - - - - - - - - ○ - 50-28-2 estradiol ○ - - - - - - - - - - - - Benzene, 1,1'-(2,2,2-trichloroethylidene)bis[4- 50-29-3 ○ ○ - - - - 388 - - - - - - chloro- 50-32-8 -
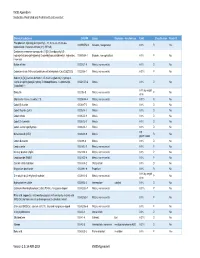
VS005 Appendix a Declarable, Restricted and Prohibited Substances List
VS005 Appendix A Declarable, Restricted and Prohibited Substances List Chemical Substance CAS RN Group Examples - function/use Limit Classification Reach CL Phosphonium, triphenyl(phenylmethyl)-, 1,1,2,2,3,3,4,4,5,5,6,6,6- 1000597-52-3 Solvents, halogenated 0.1% R Yes tridecafluoro-1-hexanesulfonate (1:1) (PFHxS) Quaternary ammonium compounds, [2-[[2-[(2-carboxyethyl)(2- hydroxyethyl)amino]ethyl]amino]-2-oxoethyl]coco alkyldimethyl, hydroxides, 100085-64-1 Biocides, non-agricultural 0.1% P No inner salts Barium nitrate 10022-31-8 Metals, non-essential 0.1% D No Cadmium nitrate (Nitric acid cadmium salt tetrahydrate (Cd ಎO3ഈ2O)) 10022-68-1 Metals, non-essential 0.01% P No Sodium [4-[[6-[(4-amino-6-chloro-1,3,5-triazin-2-yl)amino]-1-hydroxy-3- sulpho-2-naphthyl]azo]-3-hydroxy-7-nitronaphthalene-1-sulphonato(4- 100231-59-2 Metals 0.1% D No )]cobaltate(1-) 0.1% by weight Dibutyltin 1002-53-5 Metals, non-essential P No of tin Strychnidin-10-one, arsenite (1:1) 100258-44-4 Metals, non-essential 0.01% R No Cobalt(II) fluoride 10026-17-2 Metals 0.1% D No Cobalt fluoride (CoF3) 10026-18-3 Metals 0.1% D No Cobalt nitrate 10026-22-9 Metals 0.1% D No Cobalt(2+) selenite 10026-23-0 Metals 0.1% D No Cobalt sulfate heptahydrate 10026-24-1 Metals 0.1% D No 0,5 Nickel fluoride (NiF2) 10028-18-9 Metals R No μg/cm²/week Cobalt distearate 1002-88-6 Metals 0.1% D No Lead arsenite 10031-13-7 Metals, non-essential 0.1% P No Mercury bromide (HgBr) 10031-18-2 Metals, non-essential 0.1% P No Lead bromide (PbBr2) 10031-22-8 Metals, non-essential 0.1% P No Calcium