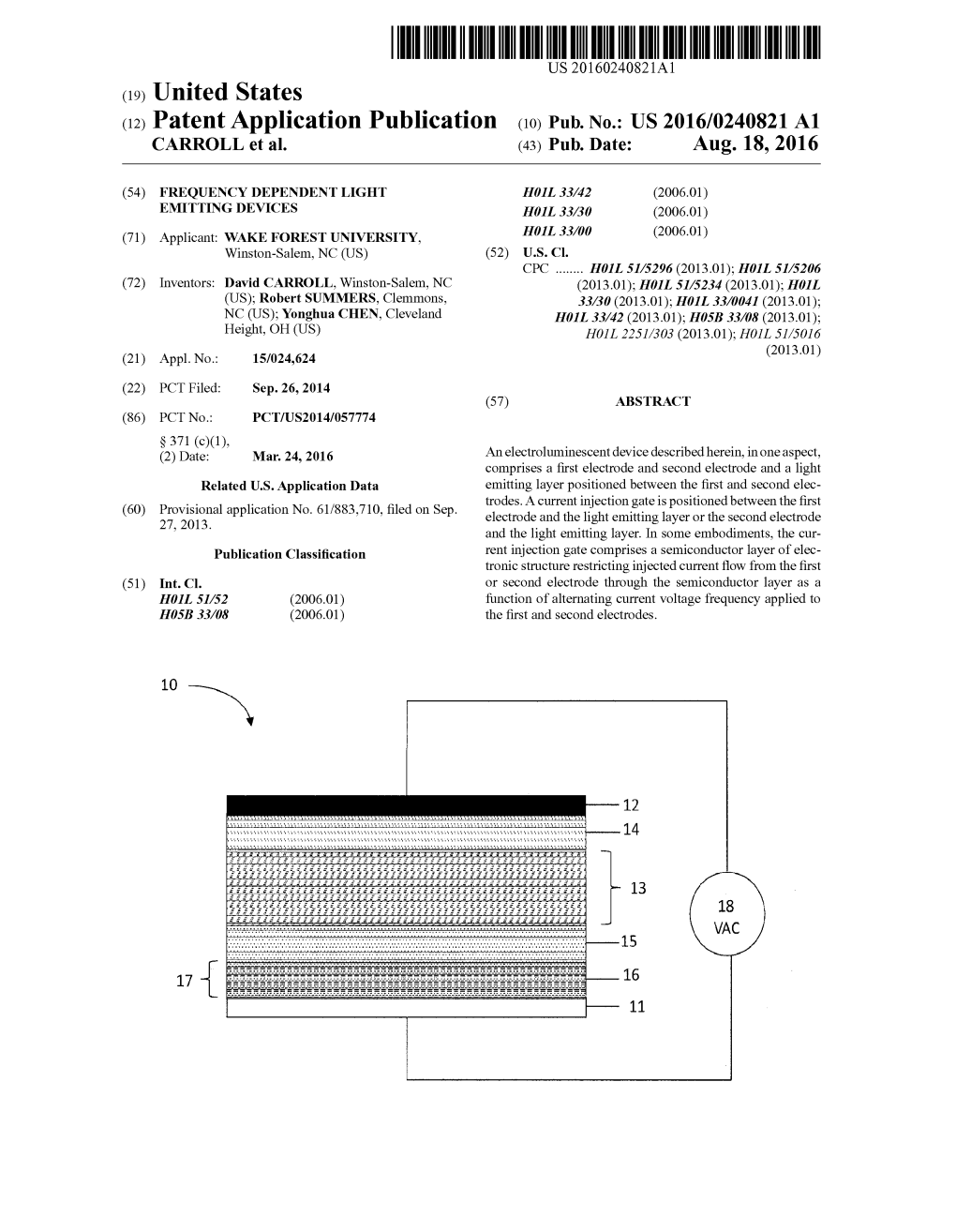
(12) Patent Application Publication (10) Pub. No.: US 2016/0240821 A1 CARROLL Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hidden Order Revealed Dilute Magnetic Semiconductors Such As Gallium Manganese Arsenide Could Be Key to the Development of Spintronics
news & views DILUTE MAGNETIC SEMICONDUCTORS Hidden order revealed Dilute magnetic semiconductors such as gallium manganese arsenide could be key to the development of spintronics. But the relationship between electronic transport and magnetic properties has been hotly debated. Data indicating the preservation of the non-magnetic character of the host material provide startling new insight. Michael E. Flatté magine striking a golf ball from a tee states may become high enough to form Ordinarily, electric current moves in the middle of a dense forest. !e ball extended-like states, contributing su&cient through ferromagnetic (Ga,Mn)As like a Iwould hit leaves, branches and trunks carriers to screen the disorder and allow golf ball that’s being continually hit through in rapid progression and not travel far. coherent charge transport similar to that a forest — slowly and incoherently. To But if the ball were hit from a tee on a associated with valence-band conduction. study the transport characteristics and platform suspended above the trees, it !e nature of the transport and electronic structure of the states in this would be free to "y considerably further. electronic structure in the prototypical material above the Fermi energy, Ohya A similar demonstration has now been dilute magnetic semiconductor (Ga,Mn) and co-workers1 constructed a series performed by Ohya et al. within the As has been the subject of lengthy debate. of resonant tunnelling diodes in which dilute magnetic semiconductor gallium At high levels of manganese doping, the carriers are injected from a non-magnetic manganese arsenide, (Ga,Mn)As, as material exhibits some extended-state semiconductor into a (Ga,Mn)As quantum reported in Nature Physics1. -

Physical Properties and Data of Optical Materials
Physical Properties and Data of Optical Materials Moriaki Wakaki Keiei Kudo Takehisa Shibuya Laß) CRC Press ^^ J Taylor &. Francis Group '*-*"' Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business • Table of Contents A AI (Aluminum) 1 AlSb (Aluminium Antimonide) 10 ADP (Ammonium Dihydrogen Phosphate) 16 Sb (Antimony) 21 Arsenic Selenium Glass 26 As (33%) + S (30%) + Br (37%) (Arsenic-Sulfur-Bromine Glass) 28 As2Se3 (Arsenic Tri-Selenide) 30 As2S3 (Arsenic Tri-Sulfide Glass) 33 6 Ba (Barium) 39 BaF2 (Barium Fluoride) 42 BaTi03 (Barium Titanate) 47 Be (Beryllium) 50 BeO (Beryllium Oxide) 54 Bi(Bismuth) 56 B(Boron) 61 C Cd(Cadmium) 65 CdSe (Cadmium Selenide) 70 CdS (Cadmium Sulfide) 75 CdTe (Cadmium Telluride) 82 CaC03 (Calcite) 89 CaF2 (Calcium Fluoride) 96 CaW04 (Calcium Tungstate) 105 CsBr (Cesium Bromide) 108 Csl (Cesium Iodide) 113 Cr(Chromium) 118 Cu(Copper) 122 CuCl (Cuprous Chloride) 128 D Diamond 135 G Ga (Gallium) 139 GaSb (Gallium Antimonide) 142 GaAs (Gallium Arsenide) 149 GaP (Gallium Phosphide) 158 Ge (Germanium) 165 Ge + Se + Te (Germanium-Selenium-Tellurium Glass) 180 Glass 182 Au (Gold) 191 * I In (Indium) 199 InSb (Indium Antimonide) 202 InAs (Indium Arsenide) 212 InP (Indium Phosphide) 218 Ir (Iridium) 224 Fe(Iron) 228 L LaF3 (Lanthanum Fluoride) 233 PbF2 (Lead Fluoride) 236 PbSe (Lead Selenide) 237 PbS (Lead Sulfide) 243 PbTe (Lead Telluride) 251 LiF (Lithium Fluoride) 257 Lucite 266 M Mg (Magnesium) 269 MgF2 (Magnesium Fluoride) 275 Mg2Ge (Magnesium Germanide) 282 MgO (Magnesium -

(12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re Et Al
US0083.33879B2 (12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re et al. (45) Date of Patent: Dec. 18, 2012 (54) ELECTRODEPOSITION OF DELECTRIC (56) References Cited COATINGS ON SEMCONDUCTIVE SUBSTRATES U.S. PATENT DOCUMENTS 3,455,806 A 7/1969 Spoor et al. (75) Inventors: Kelly L. Moore, Dunbar, PA (US); 3,663,389 A 5, 1972 Koral et al. Michael J. Pawlik, Glenshaw, PA (US); 3,749,657 A 7, 1973 Le Bras et al. Michael G. Sandala, Pittsburgh, PA 3,793,278 A 2f1974 De Bona (US); Craig A. Wilson, Allison Park, PA 3,928, 157 A 12/1975 Suematsu et al. 3,947.338 A 3, 1976 Jerabek et al. (US) 3,947,339 A 3, 1976 Jerabek et al. 3,962,165 A 6, 1976 Bosso et al. (73) Assignee: PPG Industries Ohio, Inc., Cleveland, 3,975,346 A 8, 1976 Bosso et al. OH (US) 3,984,299 A 10, 1976 Jerabek (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. EP OO12463 A1 6, 1980 (21) Appl. No.: 13/240,455 OTHER PUBLICATIONS (22) Filed: Sep. 22, 2011 Kohler, E. P. "An Apparatus for Determining Both the Quantity of Gas Evolved and the Amount of Reagent Consumed in Reactions (65) Prior Publication Data with Methyl Magnesium Iodide'. J. Am. Chem. Soc., 1927, 49 (12), US 2012/OOO6683 A1 Jan. 12, 2012 3181-3188, American Chemical Society, Washington, D.C. Related U.S. -

Safety Data Sheet
SAFETY DATA SHEET Revision Date 29-Jun-2018 Revision Number 1 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identification Product Description: Cadmium arsenide Cat No. : 22773 CAS-No 12006-15-4 Molecular Formula As2 Cd3 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Based on available data, the classification criteria are not met Health hazards Acute oral toxicity Category 3 (H301) Acute Inhalation Toxicity - Dusts and Mists Category 2 (H330) Environmental hazards ______________________________________________________________________________________________ ALFAA22773 Page 1 / 10 SAFETY DATA SHEET Cadmium arsenide Revision Date 29-Jun-2018 ______________________________________________________________________________________________ Acute aquatic toxicity Category 1 (H400) Chronic -
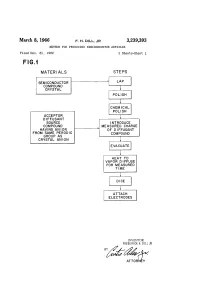
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD FOR PRODUCING SEMICONDUCTOR ARTICLES Filed Dec. 31, 1962 2. Sheets-Sheet FG. MATER ALS STEPS SEMCONDUCTOR COMPOUND CRYSTAL POLISH CHEMICAL POLISH ACCEPTOR DFFUSANT SOURCE INTRODUCE COMPOUND MEASURED CHARGE HAVING AN ON OF DFFUSANT FROM SAME PERODC COMPOUND GROUP AS CRYSTAL ANION EVACUATE HEAT TO VAPOR DIFFUSE FOR MEASURED TIME ATTACH ELECTRODES INVENTOR. FREDERICK H. DLL JR ATTORNEY March 8, 1966 F., H., DL, JR 3,239,393 METHOD FOR PRODUCING SEMCONDUCTOR ARTICLES Filed Dec. 31, 962 2. Sheets-Sheet 2 Šes is series NNNNN r N NNNNNNNN 3,239,393 United States Patent Office Patented Mar. 8, 1966 2. to produce precisely the desired results. For instance, 3,239,393 when metallic zinc is used as the diffusant material for METHOD FOR PRODUCING SEMCONDUCTOR a gallium arsenide substrate, it is very difficult to obtain ARTICLES the pure zinc metal with no zinc oxide film upon the metal. Frederick H. Dii, Jr., Patnam Waley, N.Y., assigor to 5 Furthermore, the metal is so tough that it is difficult to International Business Machines Corporation, New divide a pure metal sample into smaller pieces in order York, N.Y., a corporation of New York to obtain exactly the correct quantity for the diffusion Filed Dec. 31, 1962, Ser. No. 248,679 process. The zinc oxide on the surface of the metallic Zinc 7 Claims. (C. 48-189) diffusant material is very undesirable for a number of This invention relates to an improved diffusion process IO reasons. The oxygen is not wanted in the diffusion Vapor, for the production of Semiconductor devices, and more and the zinc oxide tends to form a protective coating particularly to an improved vapor diffusion process in over the zinc which inhibits the formation of the desired which the introduction of unwanted impurities is very zinc metal vapor which is required for the diffusion proc effectively and simply avoided, and which possesses other CSS. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Thermomagnetic Effects in Cadmium Arsenide
Thermomagnetic effects in cadmium arsenide Citation for published version (APA): Blom, F. A. P. (1970). Thermomagnetic effects in cadmium arsenide. Technische Hogeschool Eindhoven. https://doi.org/10.6100/IR155506 DOI: 10.6100/IR155506 Document status and date: Published: 01/01/1970 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Flexible Thermal Interface Based on Self-Assembled Boron Arsenide for High-Performance Thermal Management
UCLA UCLA Previously Published Works Title Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management. Permalink https://escholarship.org/uc/item/4b77z423 Journal Nature communications, 12(1) ISSN 2041-1723 Authors Cui, Ying Qin, Zihao Wu, Huan et al. Publication Date 2021-02-24 DOI 10.1038/s41467-021-21531-7 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ARTICLE https://doi.org/10.1038/s41467-021-21531-7 OPEN Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management ✉ Ying Cui1, Zihao Qin1, Huan Wu1, Man Li1 & Yongjie Hu 1 Thermal management is the most critical technology challenge for modern electronics. Recent key materials innovation focuses on developing advanced thermal interface of elec- 1234567890():,; tronic packaging for achieving efficient heat dissipation. Here, for the first time we report a record-high performance thermal interface beyond the current state of the art, based on self- assembled manufacturing of cubic boron arsenide (s-BAs). The s-BAs exhibits highly desirable characteristics of high thermal conductivity up to 21 W/m·K and excellent elastic compliance similar to that of soft biological tissues down to 100 kPa through the rational design of BAs microcrystals in polymer composite. In addition, the s-BAs demonstrates high flexibility and preserves the high conductivity over at least 500 bending cycles, opening up new application opportunities for flexible thermal cooling. Moreover, we demonstrated device integration with power LEDs and measured a superior cooling performance of s-BAs beyond the current state of the art, by up to 45 °C reduction in the hot spot temperature. -

Ferromagnetic Compounds of Manganese with Group V-A Elements
FEBBOKAGIETIC COMPOUNDS OF MANGANESE WITH GROUP V-A ELEMENTS bf lEITH HOLCOMB SWEENY A THESIS aubll1tted '<> OREGON STATE COLLEGE in partial tult1llment ot the requirements tor the degree ot DOCTOR OF PHILOSOPHY June 1951 "rttnflmr Redacted for Privacy 5f*rlf }rofrrlor d {tr*rtrf I: *rrtt of lrrr Redacted for Privacy lHfilf of thr frerrttnt d &r!,rtrf Redacted for Privacy 0hrrrrrp of SlDeol Grnnrlr 0rll,ttro Redacted for Privacy Iu}.tr *dmtr E*eol ht &d.r 1r $p.A bf lirramr hrff ACDOWLEDGMEHTS The author wishes to express his gratitude to Dr. Allen B. Scott tor hie enthuaiaat1c and untiring interest in this 1nYest1gat1on; hie helpful guidance la a1ncerelJ appreciated. To those other members or the Chem1str7 and Physics departments who have aided ln this work, a grateful thank you. A large portion or this work was conducted while the author held a fellowship given b7 the E. I. du Pont de Nemours Comp&n7. The honor and aaaiatanoe given bJ tbia tellovahip is gratetull7 acknowledged. TABLE OF CONTENTS SUBJECT PAGE IKTRODUCTION 1 ObJects 1 B. FUNDAMENTAL RELATIONSHIPS OF FERROMAGIET IC THEORY 0 Ew1ng 1 s Contribution 6 fhe Weiss Thttory . 7 Bei~te:nberg Theory of Je!"romagnet1sm 14 Exchange Integral Oonfl1'11ons 16 C. PREPARA'l'IOli OF TSE SAMPLES 22 History 22 Allo1s Produced by Direct Comb1natt.on 26 Chemical. and Electrolytic Preparation ot Al1oJa 29 Crystallization Experiments 30 Tube Furnace Method ot Preparation ot Alloye33 Analya1a or Samples 39 Rhenium- Antimony Alloy 42 D, EXPERIMENTAL 45 Temperatare Meaauremen t 46 Thermoregulator 47 Dilatometer 52 Permeameter 57 Thermoelectric Potential 62 'fhermogal1'an1c Potential 65 Sugge·ated Improvements in the Apparatus 66 E . -

Advanced Crystal Growth Techniques with Iii-V Boron Compound
ADVANCED CRYSTAL GROWTH TECHNIQUES WITH III-V BORON COMPOUND SEMICONDUCTORS by CLINTON E. WHITELEY B.S., Benedictine Collage, 2005 M.S., Kansas State University, 2008 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Department of Chemical Engineering College of Engineering KANSAS STATE UNIVERSITY Manhattan, Kansas 2011 ABSTRACT Semiconducting icosahedral boron arsenide, B12As2, is an excellent candidate for neutron detectors and radioisotope batteries, for which high quality single crystals are required. Thus, the present study was undertaken to grow B12As2 crystals by precipitation from metal solutions (nickel) saturated with elemental boron and arsenic in a sealed quartz ampoule. B12As2 crystals of 8-10 mm were produced when a homogeneous mixture of the three elements was held at 1150 °C for 48-72 hours and slowly cooled (3°C/hr). The crystals varied in color and transparency from black and opaque to clear and transparent. X-ray topography (XRT), Raman spectroscopy, and defect selective etching confirmed that the crystals had the expected rhombohedral structure and a low density of defects (5x107 cm-2). The concentrations of residual impurities (nickel, carbon, etc) were found to be relatively high (1019 cm-3 for carbon) as measured by secondary ion mass spectrometry (SIMS) and elemental analysis by energy dispersive x-ray spectroscopy (EDS). The boron arsenide crystals were found to have favorable electrical properties (µ = 24.5 cm2 / Vs), but no interaction between a prototype detector and an alpha particle bombardment was observed. Thus, the flux growth method is viable for growing large B12As2 crystals, but the impurity concentrations remain a problem. -

Boron Isotope Effect on the Thermal Conductivity of Boron Arsenide Single Crystals
Boron isotope effect on the thermal conductivity of boron arsenide single crystals Haoran Sun1, Ke Chen2, Geethal Amila Gamage1, Hamidreza Ziyaee3, Fei Wang1, Yu Wang1,4, Viktor G. Hadjiev3, Fei Tian1†, Gang Chen2, and Zhifeng Ren1† 1 Department of Physics and Texas Center for Superconductivity (TcSUH), University of Houston, Houston, Texas 77204, USA 2 Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 3 Department of Mechanical Engineering and Texas Center for Superconductivity (TcSUH), University of Houston, Houston, Texas 77204, USA 4 Institute of Advanced Materials, Hubei Normal University, Huangshi, Hubei 435002, China †To whom correspondence should be addressed, email: [email protected], [email protected] Abstract Boron arsenide (BAs) with a zinc blende structure has recently been discovered to exhibit unusual and ultrahigh thermal conductivity (k), providing a new outlook for research on BAs and other high thermal conductivity materials. Technology for BAs crystal growth has been continuously improving, however, the influence of boron isotopes, pure or mixed, on the thermal conductivity in BAs is still not completely clear. Here we report detailed studies on the growth of single crystals of BAs with different isotopic ratios and demonstrate that the k of isotopically pure BAs is at least 10% higher than that of BAs grown from natural B. Raman spectroscopy characterization shows differences in scattering among various BAs samples. The presented results will be helpful in guiding further studies on the 1 influence of isotopes on optimizing k in BAs. 2 Introduction The heat produced in high power density electronic devices imposes a major limitation on the performance of these devices. -

Cubic Boron Phosphide Epitaxy on Zirconium Diboride Balabalaji Padavala, H
Cubic boron phosphide epitaxy on zirconium diboride Balabalaji Padavala, H. Al Atabi, Lina Tengdelius, Jun Lu, Hans Högberg and J. H. Edgar The self-archived postprint version of this journal article is available at Linköping University Institutional Repository (DiVA): http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-144241 N.B.: When citing this work, cite the original publication. Padavala, B., Al Atabi, H., Tengdelius, L., Lu, J., Högberg, H., Edgar, J. H., (2018), Cubic boron phosphide epitaxy on zirconium diboride, Journal of Crystal Growth, 483, 115-120. https://doi.org/10.1016/j.jcrysgro.2017.11.014 Original publication available at: https://doi.org/10.1016/j.jcrysgro.2017.11.014 Copyright: Elsevier http://www.elsevier.com/ Cubic Boron Phosphide Epitaxy on Zirconium Diboride Balabalaji Padavala1, H. Al Atabi1, Lina Tengdelius2, Jun Lu2, Hans Högberg2 and J.H. Edgar1 Kansas State University, Department of Chemical Engineering, Durland Hall, Manhattan, KS 66506, USA Thin Film Physics Division, Department of Physics, Chemistry, and Biology (IFM), Linköping University, SE-581 83 Linköping, Sweden Abstract Cubic boron phosphide (BP) is one of the least studied III-V compound semiconductors, in part because it is difficult to prepare in high quality form. In this study, zirconium diboride (ZrB2) was studied as a potential substrate for BP epitaxial layers, because of its advantages of a low lattice constant mismatch and high thermal stability. Two types of substrates were considered: ZrB2(0001) epitaxial films on 4H- SiC(0001) and bulk ZrB2(0001) single crystals. The optimal temperature for epitaxy on these substrates was 1100 °C; higher and lower temperatures resulted in polycrystalline films.