Premature Suture Closure and Ectopic Cranial Bone in Mice Expressing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cranial Suture Mesenchymal Stem Cells: Insights and Advances
biomolecules Review Cranial Suture Mesenchymal Stem Cells: Insights and Advances Bo Li 1, Yigan Wang 1, Yi Fan 2, Takehito Ouchi 3 , Zhihe Zhao 1,* and Longjiang Li 4,* 1 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China; [email protected] (B.L.); [email protected] (Y.W.) 2 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China; [email protected] 3 Department of Physiology, Tokyo Dental College, Tokyo 1010061, Japan; [email protected] 4 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Head and Neck Oncology, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China * Correspondence: [email protected] (Z.Z.); [email protected] (L.L.) Abstract: The cranial bones constitute the protective structures of the skull, which surround and protect the brain. Due to the limited repair capacity, the reconstruction and regeneration of skull defects are considered as an unmet clinical need and challenge. Previously, it has been proposed that the periosteum and dura mater provide reparative progenitors for cranial bones homeostasis and injury repair. In addition, it has also been speculated that the cranial mesenchymal stem cells reside in the perivascular niche of the diploe, namely, the soft spongy cancellous bone between the interior and exterior layers of cortical bone of the skull, which resembles the skeletal stem cells’ distribution pattern of the long bone within the bone marrow. -

Modelling Suture Ossification: a View from the Cranial Capsule
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-1992 Modelling Suture Ossification: A View from the Cranial Capsule Hugh Bryson Matternes University of Tennessee, Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Anthropology Commons Recommended Citation Matternes, Hugh Bryson, "Modelling Suture Ossification: A View from the Cranial Capsule. " Master's Thesis, University of Tennessee, 1992. https://trace.tennessee.edu/utk_gradthes/4192 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Hugh Bryson Matternes entitled "Modelling Suture Ossification: A View from the Cranial Capsule." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Arts, with a major in Anthropology. Richard L. Jantz, Major Professor We have read this thesis and recommend its acceptance: Lyle W. Konigsberg, William M. Bass Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: -

Cranial Sutures & Funny Shaped Heads: Radiological Diagnosis
Objectives • The objectives of this presentation are to: – Review the imaging features of normal cranial sutures – Identify the characteristics of abnormal skull shape on imaging – Review the characteristics of the most common non- syndromic and syndromic causes of craniosynostosis Anatomical Review Anatomical Review • The bony plates of the skull communicate at the cranial sutures • The anterior fontanelle occurs where the coronal & metopic sutures meet • The posterior fontanelle occurs where the sagittal & lambdoid sutures meet Anatomical Review • The main cranial sutures & fontanelles include: Metopic Suture Anterior Fontanelle Coronal Sutures Squamosal Sutures Posterior Fontanelle Sagittal Suture Lambdoid Sutures Anatomical Review • Growth of the skull occurs perpendicular to the cranial suture • This is controlled by a complex signalling system including: – Ephrins (mark the suture boundary) – Fibroblast growth factor receptors (FGFR) – Transcription factor TWIST Anatomical Review • The cranial sutures are important for rapid skull growth in-utero & infancy • The cranial sutures can usually be visualised on imaging into late adulthood Normal Radiological Appearances Normal Radiological Appearances • The cranial sutures can be visualised on plain radiographs • Standard views include: – PA – Lateral – Townes PA Skull radiograph Sagittal Suture Left Coronal Suture Right CoronalRight lambdoidSutureSuture Metopic Suture Left lambdoid Suture Townes View Sagittal Suture Left Coronal Suture Right Coronal Suture Right Lambdoid Suture Left -

A Heads up on Craniosynostosis
Volume 1, Issue 1 A HEADS UP ON CRANIOSYNOSTOSIS Andrew Reisner, M.D., William R. Boydston, M.D., Ph.D., Barun Brahma, M.D., Joshua Chern, M.D., Ph.D., David Wrubel, M.D. Craniosynostosis, an early closure of the growth plates of the skull, results in a skull deformity and may result in neurologic compromise. Craniosynostosis is surprisingly common, occurring in one in 2,100 children. It may occur as an isolated abnormality, as a part of a syndrome or secondary to a systemic disorder. Typically, premature closure of a suture results in a characteristic cranial deformity easily recognized by a trained observer. it is usually the misshapen head that brings the child to receive medical attention and mandates treatment. This issue of Neuro Update will present the diagnostic features of the common types of craniosynostosis to facilitate recognition by the primary care provider. The distinguishing features of other conditions commonly confused with craniosynostosis, such as plagiocephaly, benign subdural hygromas of infancy and microcephaly, will be discussed. Treatment options for craniosynostosis will be reserved for a later issue of Neuro Update. Types of Craniosynostosis Sagittal synostosis The sagittal suture runs from the anterior to the posterior fontanella (Figure 1). Like the other sutures, it allows the cranial bones to overlap during birth, facilitating delivery. Subsequently, this suture is the separation between the parietal bones, allowing lateral growth of the midportion of the skull. Early closure of the sagittal suture restricts lateral growth and creates a head that is narrow from ear to ear. However, a single suture synostosis causes deformity of the entire calvarium. -

The Sima De Los Huesos Crania: Analysis of the Cranial Breakage Patterns
Journal of Archaeological Science 72 (2016) 25e43 Contents lists available at ScienceDirect Journal of Archaeological Science journal homepage: http://www.elsevier.com/locate/jas The Sima de los Huesos Crania: Analysis of the cranial breakage patterns * Nohemi Sala a, b, c, , Ana Pantoja-Perez c, a, d, Juan Luis Arsuaga c, d, Adrian Pablos e, f, a, c, Ignacio Martínez a, c a Area de Antropología Física, Departamento de Ciencias de la Vida, Universidad de Alcala, Madrid, Spain € b Institut für Ur- und Frühgeschichte und Archaologie€ des Mittelalters Abteilung für Altere Urgeschichte und Quartar€ okologie,€ Eberhard-Karls Universitat€ Tübingen, Schloss Hohentübingen, 72070 Tübingen, Germany c Centro Mixto UCM-ISCIII de Evolucion y Comportamiento Humanos, Madrid, Spain d Departamento de Paleontología, Facultad Ciencias Geologicas, Universidad Complutense de Madrid, Spain e Centro Nacional de Investigacion sobre Evolucion Humana-CENIEH, Burgos, Spain f Paleoanthropology, Senckenberg Center for Human Evolution and Paleoenvironment, Eberhard Karls Universitat€ Tübingen, Germany article info abstract Article history: The Sima de los Huesos (SH) site has provided the largest collection of hominin crania in the fossil record, Received 29 March 2016 offering an unprecedented opportunity to perform a complete Forensic-Taphonomic study on a popu- Received in revised form lation from the Middle Pleistocene. The fractures found in seventeen crania from SH display a post- 1 June 2016 mortem fracturation pattern, which occurred in the dry bone stage and is compatible with collective Accepted 3 June 2016 burial assemblages. Nevertheless, in addition to the postmortem fractures, eight crania also display some Available online 14 June 2016 typical perimortem traumas. By using CT images we analyzed these fractures in detail. -

Chapter 7 Body Systems
Identification and Palpation of Individual Joints of the Body 1 Joints of the Skull Cranial sutures 2 The four cranial sutures are the coronal suture, the sagittal suture, the squamous suture, and the lambdoidal suture. The coronal suture is located on the top of the skull above the eyebrows. The sagittal suture is midway between the top of the ear and the eye. The squamous suture arcs above the ear, and the lambdoidal suture is behind the ear, just above the mastoid process in an arc superiorly and posteriorly. Joints of the Skull Temporomandibular joint 4 The temporomandibular joint consists of the temporal bone and mandible and is a synovial modified hinge joint. The TMJ is one of the strongest joints in the body and is the only biarticular joint in the body. Joints of the Shoulder Glenohumeral joint 6 The glenohumeral joint is the main joint in the shoulder and the most mobile joint in the human body. The previous figure shows the ligaments of the shoulder. Joints of the Shoulder Sternoclavicular joint 8 The movements of the sternoclavicular joint follow the movements of the scapula and clavicle because no muscle works directly on this joint. A decrease or loss of mobility in this joint directly affects shoulder movement. This joint is the only direct connection between the axial skeleton and the shoulder girdle and arm. Joints of the Shoulder Acromioclavicular 10 Although a small joint, the acromioclavicular joint is important for shoulder movements. Some people do not have an acromioclavicular joint because the bones have fused. Joints of the Elbow Ulnohumeral and radiohumeral joints 12 These two joints allow flexion and extension. -
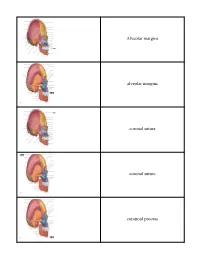
Crista Galli (Part of Cribriform Plate of Ethmoid Bone)
Alveolar margins alveolar margins coronal suture coronal suture coronoid process crista galli (part of cribriform plate of ethmoid bone) ethmoid bone ethmoid bone ethmoid bone external acoustic meatus external occipital crest external occipital protuberance external occipital protuberance frontal bone frontal bone frontal bone frontal sinus frontal squama of frontal bone frontonasal suture glabella incisive fossa inferior nasal concha inferior nuchal line inferior orbital fissure infraorbital foramen internal acoustic meatus lacrimal bone lacrimal bone lacrimal fossa lambdoid suture lambdoid suture lambdoid suture mandible mandible mandible mandibular angle mandibular condyle mandibular foramen mandibular notch mandibular ramus mastoid process of the temporal bone mastoid process of the temporal bone maxilla maxilla maxilla mental foramen mental foramen middle nasal concha of ethmoid bone nasal bone nasal bone nasal bone nasal bone occipital bone occipital bone occipital bone occipitomastoid suture occipitomastoid suture occipitomastoid suture occipital condyle optic canal optic canal palatine bone palatine process of maxilla parietal bone parietal bone parietal bone parietal bone perpendicular plate of ethmoid bone pterygoid process of sphenoid bone sagittal suture sella turcica of sphenoid bone Sphenoid bone (greater wing) spehnoid bone (greater wing) sphenoid bone (greater wing) sphenoid bone (greater wing) sphenoid sinus sphenoid sinus squamous suture squamous suture styloid process of temporal bone superior nuchal line superior orbital fissure supraorbital foramen (notch) supraorbital margin sutural bone temporal bone temporal bone temporal bone vomer bone vomer bone zygomatic bone zygomatic bone. -
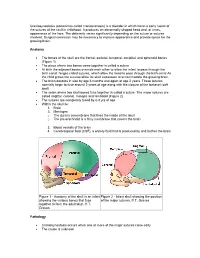
Craniosynostosis (Sometimes Called Craniostenosis) Is a Disorder in Which There Is Early Fusion of the Sutures of the Skull in Childhood
Craniosynostosis (sometimes called craniostenosis) is a disorder in which there is early fusion of the sutures of the skull in childhood. It produces an abnormally shaped head and, at times, appearance of the face. The deformity varies significantly depending on the suture or sutures involved. Surgical correction may be necessary to improve appearance and provide space for the growing brain. Anatomy • The bones of the skull are the frontal, parietal, temporal, occipital, and sphenoid bones (Figure 1) • The place where two bones come together is called a suture • At birth the adjacent bones override each other to allow the infant to pass through the birth canal. hinges called sutures, which allow the head to pass through the birth canal As the child grows the sutures allow for skull expansion to accommodate the growing brain. • The brain doubles in size by age 6 months and again at age 2 years. These sutures normally begin to fuse around 2 years of age along with the closure of the fontanel (soft spot) • The seam where two skull bones fuse together is called a suture. The major sutures are called sagittal, coronal, metopic and lambdoid (Figure 2) • The sutures are completely fused by 6-8 yrs of age • Within the skull lie: 1. Brain 2. Meninges o The dura is a membrane that lines the inside of the skull o The pia-arachnoid is a filmy membrane that covers the brain 3. Blood vessels of the brain 4. Cerebrospinal fluid (CSF), a watery fluid that is produced by and bathes the brain Figure 1 - Anatomy of the skull in an infant Figure 2 - Infant skull showing the position showing the various bones that fuse of the major sutures. -

Malpositions of the Occiput and Malpresentations
31 Malpositions of the occiput and malpresentations Terri Coates CHAPTER CONTENTS Occipitoposterior positions 574 Management of labour 593 Causes 574 Complications 599 Antenatal diagnosis 574 Shoulder presentation 600 Diagnosis during labour 576 Causes 600 Care in labour 576 Antenatal diagnosis 601 Mechanism of right occipitoposterior position Intrapartum diagnosis 601 (long rotation) 577 Possible outcome 601 Possible course and outcomes of labour 578 Management 602 Complications 581 Complications 602 Face presentation 581 Unstable lie 602 Causes 581 Causes 602 Antenatal diagnosis 582 Management 603 Intrapartum diagnosis 582 Complications 603 Mechanism of a left mentoanterior Compound presentation 603 position 583 REFERENCES 603 Possible course and outcomes of labour 584 FURTHER READING 604 Management of labour 585 Complications 585 Brow presentation 586 Causes 586 Diagnosis 586 Management 587 Complications 587 Breech presentation 587 Types of breech presentation and position 587 Causes 588 Antenatal diagnosis 589 Diagnosis during labour 589 Antenatal management 590 Persistent breech presentation 592 Mechanism of left sacroanterior position 593 573 574 LABOUR occupy the roomier hindpelvis. The oval shape of the Malpositions and malpresentations of the fetus anthropoid pelvis, with its narrow transverse diameter, present the midwife with a challenge of favours a direct occipitoposterior position. recognition and diagnosis both in the antenatal period and during labour. Antenatal diagnosis This chapter aims to: Abdominal examination • outline the causes of these positions and presentations Listen to the mother • discuss the midwife’s diagnosis and management The mother may complain of backache and she may • describe the possible outcomes. feel that her baby’s bottom is very high up against her ribs. -

Durham Research Online
Durham Research Online Deposited in DRO: 28 February 2019 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Santana, Jonathan and Rodr¡guez-Santos, Francisco Javier and Camalich-Massieu, Mar¡aDolores and Mart¡n-Socas, Dimas and Fregel, Rosa (2019) 'Aggressive or funerary cannibalism? skull-cup and human bone manipulation in Cueva de El Toro (Early Neolithic, southern Iberia).', American journal of physical anthropology., 169 (1). pp. 31-54. Further information on publisher's website: https://doi.org/10.1002/ajpa.23805 Publisher's copyright statement: This is the accepted version of the following article: Santana, Jonathan, Rodr¡guez-Santos, Francisco Javier, Camalich-Massieu, Mar¡aDolores, Mart¡n-Socas, Dimas Fregel, Rosa (2019). Aggressive or funerary cannibalism? Skull-cup and human bone manipulation in Cueva de El Toro (Early Neolithic, southern Iberia). American Journal of Physical Anthropology 169(1): 31-54, which has been published in nal form at https://doi.org/10.1002/ajpa.23805. This article may be used for non-commercial purposes in accordance With Wiley Terms and Conditions for self-archiving. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. -

Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

A Joint Or Articulation Is a Place in the Body Where Two Bones Come Together
Definition: A joint or articulation is a place in the body where two bones come together. CLASSES OF JOINTS. 1. Joints are classified according to how the bones are held together. 2. The three types of joints are fibrous, cartilaginous, and synovial. Fibrous Joints 1. The bones are held together by fibrous connective tissue and exhibit little or no movement. 2. The three types of fibrous joints are sutures, syndesmoses, and gomphoses. Sutures 1. Sutures are fibrous joints between the bones of the skull. The bones are close together and bound together by sutural ligaments. In adults, some sutures allow no movement between the bones. FIGURE 8.1 Periosteum Bone Sutural ligament 2. In the newborn, the sutures are fairly wide. In some locations, they are very large and are called fontanels. This allows the skull to "give" during delivery. The frontal bone and mandible are divided by a suture. FIGURE 8.2 3. A synostosis occurs when two bones grow together across a joint to form a single bone. A. This normally occurs around two years of age for the mandible and the frontal bone. B. Around forty years of age (there is great variability) other sutures begin to fuse. 8-1 Name a place in the body (other than the skull) where a synostosis occurs? What shape would the skull be if the sagittal suture fused prematurely before growth of the skull was completed? Syndesmoses 1. A syndesmosis is a fibrous joint that is held together by longer fibers than a suture and some movement can occur. 2.