Cranial Suture Mesenchymal Stem Cells: Insights and Advances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronal and Lambdoid Suture Evolution Following Total Vault Remodeling for Scaphocephaly
NEUROSURGICAL FOCUS Neurosurg Focus 50 (4):E4, 2021 Coronal and lambdoid suture evolution following total vault remodeling for scaphocephaly Pierre-Aurélien Beuriat, MD, PhD,1,2,4 Alexandru Szathmari, MD, PhD,1,2 Julie Chauvel-Picard, MD,1,3,4 Arnaud Gleizal, MD, PhD,1,3,4 Christian Paulus, MD,1,3 Carmine Mottolese, MD, PhD,1,2 and Federico Di Rocco, MD, PhD1,2,4 1French Referral Center for Craniosynostosis; Departments of 2Pediatric Neurosurgery and 3Pediatric Maxillo-Facial Surgery, Hôpital Femme Mère Enfant; and 4Université de Lyon, France OBJECTIVE Different types of surgical procedures are utilized to treat craniosynostosis. In most procedures, the fused suture is removed. There are only a few reports on the evolution of sutures after surgical correction of craniosynostosis. To date, no published study describes neosuture formation after total cranial vault remodeling. The objective of this study was to understand the evolution of the cranial bones in the area of coronal and lambdoid sutures that were removed for complete vault remodeling in patients with sagittal craniosynostosis. In particular, the investigation aimed to confirm the possibility of neosuture formation. METHODS CT images of the skulls of children who underwent operations for scaphocephaly at the Hôpital Femme Mère Enfant, Lyon University Hospital, Lyon, France, from 2004 to 2014 were retrospectively reviewed. Inclusion crite- ria were diagnosis of isolated sagittal synostosis, age between 4 and 18 months at surgery, and availability of reliable postoperative CT images obtained at a minimum of 1 year after surgical correction. Twenty-six boys and 11 girls were included, with a mean age at surgery of 231.6 days (range 126–449 days). -

The Role of Movements in the Development of Sutural
J. Anat. (1983), 137, 3, pp. 591-599 591 With 11 figures Printed in Great Britain The role of movements in the development of sutural and diarthrodial joints tested by long-term paralysis of chick embryos MAURITS PERSSON Department of Orthodontics, Faculty of Odontology, University of Umea, Norrlandsgatan 18 B, S-902 48 Umea, Sweden (Accepted 17 February 1983) INTRODUCTION The factors regulating the development of sutural fibrous joints are a subject of controversy. Movements of muscular origin (Pritchard, Scott & Girgis, 1956), an osteogenesis-inhibiting factor (Markens, 1975) and physiolQgical cell death (Ten Cate, Freeman & Dickinson, 1977) have been suggested. More recently, Smith & Tondury (1978) concluded that stretch-growth tensile forces in the dura mater are responsible for the development of the calvaria and its sutures, the primary deter- minant in their development being the form and growth of the early brain. A similar biomechanical explanation for the morphogenesis of sutures in areas of the skull where no dura mater exists was also given by Persson & Roy (1979). Based on studies of suture development and bony fusion in the rabbit palate, they con- cluded that the spatial separation of bones during growth is the factor regulating suture formation. Lack of such movements of developing bones results in bone fusion when the bones meet. Further, when cranial bones at suture sites are im- mobilised, fusion of the bones across the suture is produced (Persson et al. 1979). In the normal development of diarthrodial joints, skeletal muscle contractions are essential (Murray & Selby, 1930; Lelkes, 1958; Drachman & Coulombre, 1962; Murray & Drachman, 1969; Hall, 1975), and lack of muscular movements results in stiff, fused joints. -

Frontosphenoidal Synostosis: a Rare Cause of Unilateral Anterior Plagiocephaly
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Childs Nerv Syst (2007) 23:1431–1438 DOI 10.1007/s00381-007-0469-4 ORIGINAL PAPER Frontosphenoidal synostosis: a rare cause of unilateral anterior plagiocephaly Sandrine de Ribaupierre & Alain Czorny & Brigitte Pittet & Bertrand Jacques & Benedict Rilliet Received: 30 March 2007 /Published online: 22 September 2007 # Springer-Verlag 2007 Abstract Conclusion Frontosphenoidal synostosis must be searched Introduction When a child walks in the clinic with a in the absence of a coronal synostosis in a child with unilateral frontal flattening, it is usually associated in our anterior unilateral plagiocephaly, and treated surgically. minds with unilateral coronal synostosis. While the latter might be the most common cause of anterior plagiocephaly, Keywords Craniosynostosis . Pediatric neurosurgery. it is not the only one. A patent coronal suture will force us Anterior plagiocephaly to consider other etiologies, such as deformational plagio- cephaly, or synostosis of another suture. To understand the mechanisms underlying this malformation, the development Introduction and growth of the skull base must be considered. Materials and methods There have been few reports in the Harmonious cranial growth is dependent on patent sutures, literature of isolated frontosphenoidal suture fusion, and and any craniosynostosis might lead to an asymmetrical we would like to report a series of five cases, as the shape of the skull. The anterior skull base is formed of recognition of this entity is important for its treatment. different bones, connected by sutures, fusing at different ages. The frontosphenoidal suture extends from the end of Presented at the Consensus Conference on Pediatric Neurosurgery, the frontoparietal suture, anteriorly and inferiorly in the Rome, 1–2 December 2006. -

Cleidocranial Dysplasia- a Case Report
Case Report Cleidocranial Dysplasia- A Case Report Akhilanand Chaurasia Department of Oral Medicine & Radiology, Faculty of Dental Sciences, King George Medical University, Lucknow E-mail: [email protected] ABSTRACT Cleidocranial dysplasia is a rare congenital disease. It is characterized by autosomal dominant inheritance pattern which is caused due to mutations in the Cbfa1 gene (Runx2) located on chromosome 6p21. It primarily affects bones which are formedby intra-membranous ossification and have equal sex distribution. It is also known as Marie and Sainton disease, Mutational dysostosis and cleidocranialdysostosis. The skeletal deformities of cleidocranial dysplasia are characterized by partial or complete absence of clavicles, late closure of the fontanels, presence of open skull sutures and multiple wormian bones. This rare syndrome is of utmost importance in dentistry due to presence of multiple supernumerary teeth, facial bones deformities and deranged eruption patterns. We are reporting a classical case of cleidocranial dysplasia in 20 year old patient. Keywords: Cleidocranial dysplasia, Marie and Sainton disease, Mutational dysostosis, Cleidocranialdysostosis, Autosomal dominant Access this article online mandibular symphysis are less common findings of Quick Response cleidocranial dysostosis1,10,11. Code: Website: Dental findings in cleidocranialdysostosis are www.innovativepublication.com characterized by a decreased eruptive force of both primary and permanent dentition, prolonged retention of primary teeth12 and an increase in odontogenesis DOI: 10.5958/2395-6194.2015.00009.0 leading to an excessive number of supernumerary teeth13. The clinical findings of cleidocranialdysostosis although present at birth are often either missed or INTRODUCTION diagnosed at a much later time. Cleidocranialdysostosis Cleidocranialdysostosis is a rare congenital defect may be identified by family history, excessive mobility primarily affecting bones which undergo intra- of shoulders and radiographic pathognomonic findings membranous ossification i.e. -

Morphological and Topographical Study of Wormian Bones in Cadaver Dry Skulls
Original article Morphological and topographical study of Wormian bones in cadaver dry skulls Murlimanju, BV.*, Prabhu, LV., Ashraf, CM., Kumar, CG., Rai, R. and Maheshwari, C. Department of Anatomy, Manipal University, Centre for Basic Sciences, Kasturba Medical College, Mangalore, India *E-mail: [email protected] Abstract Introduction: The Wormian bones are formations associated with insufficient rate of suture closure and regarded as epigenetic and hypostotic traits. It was reported that there exists racial variability among the incidence of these bones. In the present study, the aims were to find the incidence of Wormian bones in Indian skulls and to analyze them topographically. Material and methods: The study included 78 human adult dry skulls of Indian population which were obtained from the neuroanatomy laboratory of our institution. They were macroscopically observed for the incidence and topographical distribution of the Wormian bones. Results: The Wormian bones were observed in 57 skulls (73.1%) of our series. Remaining 21 skulls (26.9%) didn’t show these variant bones. They were observed at the lambdoid suture in 56.4% cases (44 skulls; 14-bilateral; 18-right side; 12-left side), at the asterion in 17.9% (14 skulls; 3-bilateral; 2-right side; 9-left side), at the pterion in 11.5% (9 skulls; 4-right side; 5-left side), at the coronal suture in 1.3% (only one skull) and at the sagittal suture in 1.3% cases (only one skull). Conclusion: The current study observed Wormian bones in 73.1% of the cases from Indian population. This incidence rate is slightly higher compared to other reports and may be due to racial variations. -

Chapter 22 TEMPORAL BONE FRACTURES
Temporal Bone Fractures Chapter 22 TEMPORAL BONE FRACTURES † CARLOS R. ESQUIVEL, MD,* AND NICHOLAS J. SCALZITTI, MD INTRODUCTION INITIAL EVALUATION FRACTURE CLASSIFICATION RADIOGRAPHIC EVALUATION MANAGEMENT OF INJURIES Facial Nerve Injury Hearing Loss Cerebrospinal Fluid Leak and Use of Antibiotic Therapy SPECIAL CONSIDERATIONS FOR WARTIME INJURIES Acute Management CASE PRESENTATIONS Case Study 22-1 Case Study 22-2 *Lieutenant Colonel (Retired), Medical Corps, US Air Force; Clinical Director, Department of Defense, Hearing Center of Excellence, Wilford Hall Ambulatory Surgical Center, 59th Medical Wing, Lackland Air Force Base, Texas 78236 †Captain, Medical Corps, US Air Force; Resident Otolaryngologist, San Antonio Military Medical Center, 3551 Roger Brooke Drive, Joint Base San Antonio, Fort Sam Houston, Texas 78234 267 Otolaryngology/Head and Neck Combat Casualty Care INTRODUCTION The conflicts in Iraq and Afghanistan have resulted age to the deeper structures of the middle and inner in large numbers of head and neck injuries to NATO ear, as well as the brain. Special arrangement of the (North Atlantic Treaty Organization) and Afghan ser- outer and middle ear is essential for efficient capture vice members. Improvised explosive devices, mortars, and transduction of sound energy to the inner ear in and suicide bombs are the weapons of choice during order for hearing to take place. This efficiency relies this conflict. The resultant injuries are high-velocity on a functional anatomical relationship of a taut tym- projectile injuries. Relatively little is known about the panic membrane connected to a mobile, intact ossicular precise incidence and prevalence of isolated closed chain. Traumatic forces that disrupt this relationship temporal bone fractures in theater. -
Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Morphology of the Pterion in Serbian Population
Int. J. Morphol., 38(4):820-824, 2020. Morphology of the Pterion in Serbian Population Morfología del Pterion en Población Serbia Knezi Nikola1; Stojsic Dzunja Ljubica1; Adjic Ivan2; Maric Dusica1 & Pupovac Nikolina4 KNEZI, N.; STOJSIC, D. L.; ADJIC, I.; MARIC, D. & PUPOVAC, N. Morphology of the pterion in Serbian population. Int. J. Morphol., 38(4):820-824, 2020. SUMMARY: The pterion is a topographic point on the lateral aspect of the skull where frontal, sphenoid, parietal and temporal bones form the H or K shaped suture. This is an important surgical point for the lesions in anterior and middle cranial fossa. This study was performed on 50 dry skulls from Serbian adult individuals from Department of Anatomy, Faculty of Medicine in Novi Sad. The type of the pterion on both sides of each skull was determined and they are calcified in four types (sphenoparietal, frontotemporal, stellate and epipteric). The distance between the center of the pterion and defined anthropological landmarks were measured using the ImageJ software. Sphenoparietal type is predominant with 86 % in right side and 88 % in left side. In male skulls, the distance from the right pterion to the frontozygomatic suture is 39.89±3.85 mm and 39.67±4.61 mm from the left pterion to the frontozygomatic suture. In female skulls the distance is 37.38±6.38 mm on the right and 35.94±6.46 mm on the left. The shape and the localization of the pterion are important because it is an anatomical landmark and should be used in neurosurgery, traumatology and ophthalmology. -
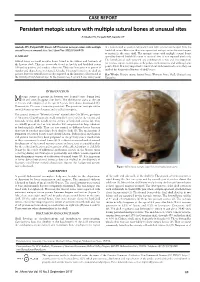
Persistent Metopic Suture with Multiple Sutural Bones at Unusual Sites
CASE REPORT Persistent metopic suture with multiple sutural bones at unusual sites Ambade HV, Fulpatil MP, Kasote AP Ambade HV, Fulpatil MP, Kasote AP. Persistent metopic suture with multiple in a human skull at asterion, left pterion and right coronal suture apart from the sutural bones at unusual sites. Int J Anat Var. 2017;10(3):69-70. lambdoid suture. Moreover, there was a persistent metopic suture between bregma to nasion in the same skull. The metopic suture with multiple sutural bones SUMMARY spreading beyond lambdoid suture at unusual sites is not reported previously. The knowledge of such variation and combination is rare and very important Sutural bones are small irregular bones found in the sutures and fontanels of for forensic expert, radiologists, orthopedists, neurosurgeons and anthropologist the human skull. They are commonly found at lambda and lambdoid suture point of view. It is very important to know about such variation because they can followed by pterion; and rarely at other sites. They vary from person to person in mislead the diagnosis of fracture of skull bones. number and shape, hence not named. Usually, 1-3 sutural bones in one skull are present, but 8-10 sutural bones are also reported in the literature, all restricted in Key Words: Metopic suture; Sutural bones; Wormian bones; Skull; Unusual sites; the vicinity of lambdoid sutures. In the present case, 8 sutural bones were present Variations INTRODUCTION etopic suture is present in between two frontal bones during fetal Mlife and soon disappear after birth. The obliteration starts at the age of 2 years and completed at the age of 8 years from above downwards (1). -

Spontaneous Encephaloceles of the Temporal Lobe
Neurosurg Focus 25 (6):E11, 2008 Spontaneous encephaloceles of the temporal lobe JOSHUA J. WIND , M.D., ANTHONY J. CAPUTY , M.D., AND FABIO ROBE R TI , M.D. Department of Neurological Surgery, George Washington University, Washington, DC Encephaloceles are pathological herniations of brain parenchyma through congenital or acquired osseus-dural defects of the skull base or cranial vault. Although encephaloceles are known as rare conditions, several surgical re- ports and clinical series focusing on spontaneous encephaloceles of the temporal lobe may be found in the otological, maxillofacial, radiological, and neurosurgical literature. A variety of symptoms such as occult or symptomatic CSF fistulas, recurrent meningitis, middle ear effusions or infections, conductive hearing loss, and medically intractable epilepsy have been described in patients harboring spontaneous encephaloceles of middle cranial fossa origin. Both open procedures and endoscopic techniques have been advocated for the treatment of such conditions. The authors discuss the pathogenesis, diagnostic assessment, and therapeutic management of spontaneous temporal lobe encepha- loceles. Although diagnosis and treatment may differ on a case-by-case basis, review of the available literature sug- gests that spontaneous encephaloceles of middle cranial fossa origin are a more common pathology than previously believed. In particular, spontaneous cases of posteroinferior encephaloceles involving the tegmen tympani and the middle ear have been very well described in the medical literature. -

Supplementary Information For
Supplementary Information for An Abundance of Developmental Anomalies and Abnormalities in Pleistocene People Erik Trinkaus Department of Anthropology, Washington University, Saint Louis MO 63130 Corresponding author: Erik Trinkaus Email: [email protected] This PDF file includes: Supplementary text Figures S1 to S57 Table S1 References 1 to 421 for SI reference citations Introduction Although they have been considered to be an inconvenience for the morphological analysis of human paleontological remains, it has become appreciated that various pathological lesions and other abnormalities or rare variants in human fossil remains might provide insights into Pleistocene human biology and behavior (following similar trends in Holocene bioarcheology). In this context, even though there were earlier paleopathological assessments in monographic treatments of human remains (e.g., 1-3), it has become common to provide details on abnormalities in primary descriptions of human fossils (e.g., 4-12), as well as assessments of specific lesions on known and novel remains [see references in Wu et al. (13, 14) and below]. These works have been joined by doctoral dissertation assessments of patterns of Pleistocene human lesions (e.g., 15-18). The paleopathological attention has been primarily on the documentation and differential diagnosis of the abnormalities of individual fossil remains, leading to the growing paleopathological literature on Pleistocene specimens and their lesions. There have been some considerations of the overall patterns of the lesions, but those assessments have been concerned primarily with non-specific stress indicators and traumatic lesions (e.g., 13, 15, 19-21), with variable considerations of issues of survival 1 w ww.pnas.org/cgi/doi/10.1073/pnas.1814989115 and especially the inferred social support of the afflicted (e.g., 22-27). -

Ectocranial Suture Closure in Pan Troglodytes and Gorilla Gorilla: Pattern and Phylogeny James Cray Jr.,1* Richard S
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 136:394–399 (2008) Ectocranial Suture Closure in Pan troglodytes and Gorilla gorilla: Pattern and Phylogeny James Cray Jr.,1* Richard S. Meindl,2 Chet C. Sherwood,3 and C. Owen Lovejoy2 1Department of Anthropology, University of Pittsburgh, Pittsburgh, PA 15260 2Department of Anthropology and Division of Biomedical Sciences, Kent State University, Kent, OH 44242 3Department of Anthropology, The George Washington University, Washington, DC 20052 KEY WORDS cranial suture; synostosis; variation; phylogeny; Guttman analysis ABSTRACT The order in which ectocranial sutures than either does with G. gorilla, we hypothesized that this undergo fusion displays species-specific variation among phylogenetic relationship would be reflected in the suture primates. However, the precise relationship between suture closure patterns of these three taxa. Results indicated that closure and phylogenetic affinities is poorly understood. In while all three species do share a similar lateral-anterior this study, we used Guttman Scaling to determine if the closure pattern, G. gorilla exhibits a unique vault pattern, modal progression of suture closure differs among Homo which, unlike humans and P. troglodyte s, follows a strong sapiens, Pan troglodytes,andGorilla gorilla.BecauseDNA posterior-to-anterior gradient. P. troglodytes is therefore sequence homologies strongly suggest that P. tr og lodytes more like Homo sapiens in suture synostosis. Am J Phys and Homo sapiens share a more recent common ancestor Anthropol 136:394–399, 2008. VC 2008 Wiley-Liss, Inc. The biological basis of suture synostosis is currently Morriss-Kay et al. (2001) found that maintenance of pro- poorly understood, but appears to be influenced by a liferating osteogenic stem cells at the margins of mem- combination of vascular, hormonal, genetic, mechanical, brane bones forming the coronal suture requires FGF and local factors (see review in Cohen, 1993).