Printer Emulator for Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Response of Marine Food Webs to Climate-Induced Changes in Temperature and Inflow of Allochthonous Organic Matter
Response of marine food webs to climate-induced changes in temperature and inflow of allochthonous organic matter Rickard Degerman Department of Ecology and Environmental Science 901 87 Umeå Umeå 2015 1 Copyright©Rickard Degerman ISBN: 978-91-7601-266-6 Front cover illustration by Mats Minnhagen Printed by: KBC Service Center, Umeå University Umeå, Sweden 2015 2 Tillägnad Maria, Emma och Isak 3 Table of Contents Abstract 5 List of papers 6 Introduction 7 Aquatic food webs – different pathways Food web efficiency – a measure of ecosystem function Top predators cause cascade effects on lower trophic levels The Baltic Sea – a semi-enclosed sea exposed to multiple stressors Varying food web structures Climate-induced changes in the marine ecosystem Food web responses to increased temperature Responses to inputs of allochthonous organic matter Objectives 14 Material and Methods 14 Paper I Paper II and III Paper IV Results and Discussion 18 Effect of temperature and nutrient availability on heterotrophic bacteria Influence of food web length and labile DOC on pelagic productivity and FWE Consequences of changes in inputs of ADOM and temperature for pelagic productivity and FWE Control of pelagic productivity, FWE and ecosystem trophic balance by colored DOC Conclusion and future perspectives 21 Author contributions 23 Acknowledgements 23 Thanks 24 References 25 4 Abstract Global records of temperature show a warming trend both in the atmosphere and in the oceans. Current climate change scenarios indicate that global temperature will continue to increase in the future. The effects will however be very different in different geographic regions. In northern Europe precipitation is projected to increase along with temperature. -

Microbial Loop' in Stratified Systems
MARINE ECOLOGY PROGRESS SERIES Vol. 59: 1-17, 1990 Published January 11 Mar. Ecol. Prog. Ser. 1 A steady-state analysis of the 'microbial loop' in stratified systems Arnold H. Taylor, Ian Joint Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth PLl 3DH, United Kingdom ABSTRACT. Steady state solutions are presented for a simple model of the surface mixed layer, which contains the components of the 'microbial loop', namely phytoplankton, picophytoplankton, bacterio- plankton, microzooplankton, dissolved organic carbon, detritus, nitrate and ammonia. This system is assumed to be in equilibrium with the larger grazers present at any time, which are represented as an external mortality function. The model also allows for dissolved organic nitrogen consumption by bacteria, and self-grazing and mixotrophy of the microzooplankton. The model steady states are always stable. The solution shows a number of general properties; for example, biomass of each individual component depends only on total nitrogen concentration below the mixed layer, not whether the nitrogen is in the form of nitrate or ammonia. Standing stocks and production rates from the model are compared with summer observations from the Celtic Sea and Porcupine Sea Bight. The agreement is good and suggests that the system is often not far from equilibrium. A sensitivity analysis of the model is included. The effect of varying the mixing across the pycnocline is investigated; more intense mixing results in the large phytoplankton population increasing at the expense of picophytoplankton, micro- zooplankton and DOC. The change from phytoplankton to picophytoplankton dominance at low mixing occurs even though the same physiological parameters are used for both size fractions. -
Suisun Marsh Fish Report 2015 Final
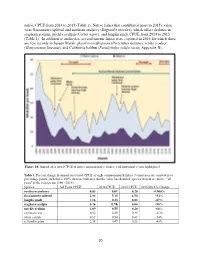
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Developments in Aquatic Microbiology
INTERNATL MICROBIOL (2000) 3: 203–211 203 © Springer-Verlag Ibérica 2000 REVIEW ARTICLE Samuel P. Meyers Developments in aquatic Department of Oceanography and Coastal Sciences, Louisiana State University, microbiology Baton Rouge, LA, USA Received 30 August 2000 Accepted 29 September 2000 Summary Major discoveries in marine microbiology over the past 4-5 decades have resulted in the recognition of bacteria as a major biomass component of marine food webs. Such discoveries include chemosynthetic activities in deep-ocean ecosystems, survival processes in oligotrophic waters, and the role of microorganisms in food webs coupled with symbiotic relationships and energy flow. Many discoveries can be attributed to innovative methodologies, including radioisotopes, immunofluores- cent-epifluorescent analysis, and flow cytometry. The latter has shown the key role of marine viruses in marine system energetics. Studies of the components of the “microbial loop” have shown the significance of various phagotrophic processes involved in grazing by microinvertebrates. Microbial activities and dissolved organic carbon are closely coupled with the dynamics of fluctuating water masses. New biotechnological approaches and the use of molecular biology techniques still provide new and relevant information on the role of microorganisms in oceanic and estuarine environments. International interdisciplinary studies have explored ecological aspects of marine microorganisms and their significance in biocomplexity. Studies on the Correspondence to: origins of both life and ecosystems now focus on microbiological processes in the Louisiana State University Station. marine environment. This paper describes earlier and recent discoveries in marine Post Office Box 19090-A. Baton Rouge, LA 70893. USA (aquatic) microbiology and the trends for future work, emphasizing improvements Tel.: +1-225-3885180 in methodology as major catalysts for the progress of this broadly-based field. -

Crangon Franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Crangon franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Caridea Common gray shrimp Family: Crangonoidea, Crangonidae Taxonomy: Schmitt (1921) described many duncle segment (Wicksten 2011). Inner fla- shrimp in the genus Crago (e.g. Crago fran- gellum of the first antenna is greater than ciscorum) and reserved the genus Crangon twice as long as the outer flagellum (Kuris et for the snapping shrimp (now in the genus al. 2007) (Fig. 2). Alpheus). In 1955–56, the International Mouthparts: The mouth of decapod Commission on Zoological Nomenclature crustaceans comprises six pairs of appendag- formally reserved the genus Crangon for the es including one pair of mandibles (on either sand shrimps only. Recent taxonomic de- side of the mouth), two pairs of maxillae and bate revolves around potential subgeneric three pairs of maxillipeds. The maxillae and designation for C. franciscorum (C. Neocran- maxillipeds attach posterior to the mouth and gon franciscorum, C. franciscorum francis- extend to cover the mandibles (Ruppert et al. corum) (Christoffersen 1988; Kuris and Carl- 2004). Third maxilliped setose and with exo- ton 1977; Butler 1980; Wicksten 2011). pod in C. franciscorum and C. alaskensis (Wicksten 2011). Description Carapace: Thin and smooth, with a Size: Average body length is 49 mm for single medial spine (compare to Lissocrangon males and 68 mm for females (Wicksten with no gastric spines). Also lateral (Schmitt 2011). 1921) (Fig. 1), hepatic, branchiostegal and Color: White, mottled with small black spots, pterygostomian spines (Wicksten 2011). giving gray appearance. Rostrum: Rostrum straight and up- General Morphology: The body of decapod turned (Crangon, Kuris and Carlton 1977). -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Controls and Structure of the Microbial Loop
Controls and Structure of the Microbial Loop A symposium organized by the Microbial Oceanography summer course sponsored by the Agouron Foundation Saturday, July 1, 2006 Asia Room, East-West Center, University of Hawaii Symposium Speakers: Peter J. leB Williams (University of Bangor, Wales) David L. Kirchman (University of Delaware) Daniel J. Repeta (Woods Hole Oceanographic Institute) Grieg Steward (University of Hawaii) The oceans constitute the largest ecosystems on the planet, comprising more than 70% of the surface area and nearly 99% of the livable space on Earth. Life in the oceans is dominated by microbes; these small, singled-celled organisms constitute the base of the marine food web and catalyze the transformation of energy and matter in the sea. The microbial loop describes the dynamics of microbial food webs, with bacteria consuming non-living organic matter and converting this energy and matter into living biomass. Consumption of bacteria by predation recycles organic matter back into the marine food web. The speakers of this symposium will explore the processes that control the structure and functioning of microbial food webs and address some of these fundamental questions: What aspects of microbial activity do we need to measure to constrain energy and material flow into and out of the microbial loop? Are we able to measure bacterioplankton dynamics (biomass, growth, production, respiration) well enough to edu/agouroninstitutecourse understand the contribution of the microbial loop to marine systems? What factors control the flow of material and energy into and out of the microbial loop? At what scales (space and time) do we need to measure processes controlling the growth and metabolism of microorganisms? How does our knowledge of microbial community structure and diversity influence our understanding of the function of the microbial loop? Program: 9:00 am Welcome and Introductory Remarks followed by: Peter J. -

Bering Sea Marine Invasive Species Assessment Alaska Center for Conservation Science
Bering Sea Marine Invasive Species Assessment Alaska Center for Conservation Science Scientific Name: Palaemon macrodactylus Phylum Arthropoda Common Name oriental shrimp Class Malacostraca Order Decapoda Family Palaemonidae Z:\GAP\NPRB Marine Invasives\NPRB_DB\SppMaps\PALMAC.pn g 40 Final Rank 49.87 Data Deficiency: 3.75 Category Scores and Data Deficiencies Total Data Deficient Category Score Possible Points Distribution and Habitat: 20 26 3.75 Anthropogenic Influence: 6.75 10 0 Biological Characteristics: 20.5 30 0 Impacts: 0.75 30 0 Figure 1. Occurrence records for non-native species, and their geographic proximity to the Bering Sea. Ecoregions are based on the classification system by Spalding et al. (2007). Totals: 48.00 96.25 3.75 Occurrence record data source(s): NEMESIS and NAS databases. General Biological Information Tolerances and Thresholds Minimum Temperature (°C) 2 Minimum Salinity (ppt) 0.7 Maximum Temperature (°C) 33 Maximum Salinity (ppt) 51 Minimum Reproductive Temperature (°C) NA Minimum Reproductive Salinity (ppt) 3 Maximum Reproductive Temperature (°C) NA Maximum Reproductive Salinity (ppt) 34 Additional Notes Palaemon macrodactylus is commonly known as the Oriental shrimp. Its body is transparent with a reddish hue in the tail fan and antennary area. Females tend to be larger than males and have more pigmentation, with reddish spots all over their body, and a whitish longitudinal stripe that runs along the back. Females reach a maximum size of 45-70 mm, compared to 31.5-45 mm for males (Vazquez et al. 2012, qtd. in Fofnoff et al. 2003). Report updated on Wednesday, December 06, 2017 Page 1 of 13 1. -

01 Delong 2-8 6/9/05 8:58 AM Page 2
01 Delong 2-8 6/9/05 8:58 AM Page 2 INSIGHT REVIEW NATURE|Vol 437|15 September 2005|doi:10.1038/nature04157 Genomic perspectives in microbial oceanography Edward F. DeLong1 and David M. Karl2 The global ocean is an integrated living system where energy and matter transformations are governed by interdependent physical, chemical and biotic processes. Although the fundamentals of ocean physics and chemistry are well established, comprehensive approaches to describing and interpreting oceanic microbial diversity and processes are only now emerging. In particular, the application of genomics to problems in microbial oceanography is significantly expanding our understanding of marine microbial evolution, metabolism and ecology. Integration of these new genome-enabled insights into the broader framework of ocean science represents one of the great contemporary challenges for microbial oceanographers. Marine ecosystems are complex and dynamic. A mechanistic under- greatly aid in these efforts. The correlation between organism- and habi- standing of the susceptibility of marine ecosystems to global environ- tat-specific genomic features and other physical, chemical and biotic mental variability and climate change driven by greenhouse gases will variables has the potential to refine our understanding of microbial and require a comprehensive description of several factors. These include biogeochemical process in ocean systems. marine physical, chemical and biological interactions including All these advances — improved cultivation, environmental genomic thresholds, negative and positive feedback mechanisms and other approaches and in situ microbial observatories — promise to enhance nonlinear interactions. The fluxes of matter and energy, and the our understanding of the living ocean system. Below, we provide a brief microbes that mediate them, are of central importance in the ocean, recent history of marine microbiology and outline some of the recent yet remain poorly understood. -

Microbial Loop Carbon Cycling in Ocean Environments Studied Using a Simple Steady-State Model
AQUATIC MICROBIAL ECOLOGY Vol. 26: 37–49, 2001 Published October 26 Aquat Microb Ecol Microbial loop carbon cycling in ocean environments studied using a simple steady-state model Thomas R. Anderson1,*, Hugh W. Ducklow2 1Southampton Oceanography Centre, Waterfront Campus, European Way, Southampton SO14 3ZH, United Kingdom 2College of William and Mary School of Marine Science, Rte 1208, Box 1346, Gloucester Point, Virginia 23062, USA ABSTRACT: A simple steady-state model is used to examine the microbial loop as a pathway for organic C in marine systems, constrained by observed estimates of bacterial to primary production ratio (BP:PP) and bacterial growth efficiency (BGE). Carbon sources (primary production including extracellular release of dissolved organic carbon, DOC), cycling via zooplankton grazing and viral lysis, and sinks (bacterial and zooplankton respiration) are represented. Model solutions indicate that, at least under near steady-state conditions, recent estimates of BP:PP of about 0.1 to 0.15 are consistent with reasonable scenarios of C cycling (low BGE and phytoplankton extracellular release) at open ocean sites such as the Sargasso Sea and subarctic North Pacific. The finding that bacteria are a major (50%) sink for primary production is shown to be consistent with the best estimates of BGE and dissolved organic matter (DOM) production by zooplankton and phytoplankton. Zooplank- ton-related processes are predicted to provide the greatest supply of DOC for bacterial consumption. The bacterial contribution to C flow in the microbial loop, via bacterivory and viral lysis, is generally low, as a consequence of low BGE. Both BP and BGE are hard to quantify accurately. -

The Role of Bacterioplankton in Lake Erie Ecosystem Processes: Phosphorus Dynamics and Bacterial Bioenergetics
THE ROLE OF BACTERIOPLANKTON IN LAKE ERIE ECOSYSTEM PROCESSES: PHOSPHORUS DYNAMICS AND BACTERIAL BIOENERGETICS A dissertation submitted to Kent State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy by Tracey Trzebuckowski Meilander December 2006 Dissertation written by Tracey Trzebuckowski Meilander B.S., The Ohio State University, 1994 M.Ed., The Ohio State University, 1997 Ph.D., Kent State University, 2006 Approved by __Robert T. Heath___________________, Chair, Doctoral Dissertation Committee __Mark W, Kershner_________________, Members, Doctoral Dissertation Committee __Laura G. Leff_____________________ __Alison J. Smith____________________ __Frederick Walz____________________ Accepted by __James L. Blank_____________________, Chair, Department of Biological Sciences __John R.D. Stalvey___________________, Dean, College of Arts and Sciences ii TABLE OF CONTENTS Page LIST OF FIGURES ………………………….……………………………………….….xi LIST OF TABLES ……………………………………………………………………...xvi DEDICATION …………………………………………………………………………..xx ACKNOWLEDGEMENTS ………………………………………………………….…xxi CHAPTER I. Introduction ….….………………………………………………………....1 The role of bacteria in aquatic ecosystems ……………………………………………….1 Introduction ……………………………………………………………………….1 The microbial food web …………………………………………………………..2 Bacterial bioenergetics ……………………………………………………………6 Bacterial productivity ……………………………………………………..6 Bacterial respiration ……………………………………………………..10 Bacterial growth efficiency ………...……………………………………11 Phosphorus in aquatic ecosystems ………………………………………………………12 -

Download Case
FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT SAN LUIS & DELTA-MENDOTA WATER AUTHORITY; WESTLANDS WATER DISTRICT, Plaintiffs-Appellants, and PIXLEY IRRIGATION DISTRICT; LOWER TULE RIVER IRRIGATION DISTRICT; TRI-VALLEY WATER dISTRICT; HILLS VALLEY IRRIGATION DISTRICT; KERN TULARE WATER DISTRICT; RAG GULCH WATER DISTRICT; STOCKTON EAST WATER DISTRICT; FRESNO COUNTY; TULARE COUNTY, Plaintiffs-Intervenors, and BAY INSTITUTE OF SAN FRANCISCO; SAVE SAN FRANCISCO BAY ASSOCIATION; ENVIRONMENTAL DEFENSE FUND; NATURAL RESOURCES DEFENSE COUNCIL; PACIFIC COAST FEDERATION OF FISHERMEN’S ASSOCIATIONS; INSTITUTE FOR FISHERIES RESOURCES; UNITED ANGLERS oF CALIFORNIA, Plaintiffs-Appellees, v. 2269 2270 SAN LUIS v. U.S. DEPARTMENT OF THE INTERIOR UNITED STATES OF AMERICA, DEPARTMENT OF THE INTERIOR, BUREAU OF RECLAMATION; KEN SALAZAR, SECRETARY OF THE INTERIOR; ROBYN THORSON, No. 09-17594 REGIONAL DIRECTOR OF THE UNITED D.C. No. STATES DEPARTMENT OF THE CV 97-06140- INTERIOR FISH AND WILDLIFE OWW SERVICE, REGION 1; DONALD GLASER, REGIONAL DIRECTOR, OPINION UNITED STATES DEPARTMENT OF THE INTERIOR BUREAU OF RECLAMATION, MID-PACIFIC REGION, Defendants-Appellees. Appeal from the United States District Court for the Eastern District of California Oliver W. Wanger, United States District Judge, Presiding Argued and Submitted March 15, 2011—Davis, California Filed March 2, 2012 Before: William A. Fletcher and Milan D. Smith, Jr., Circuit Judges, and George H. Wu, District Judge.* Opinion by Judge Wu; Partial Concurrence and Partial Dissent by Judge M. Smith *The Honorable George H. Wu, United States District Judge for the Central District of California, sitting by designation. 2274 SAN LUIS v. U.S. DEPARTMENT OF THE INTERIOR COUNSEL Thomas W.