1 Checklist of the Shrimps, Crabs, Lobsters and Crayfish of British Columbia 2011 (Order Decapoda) by Aaron Baldwin, Phd Candida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

The Tail Flip Escape Response of the Brown Shrimp Crangon Crangon (L.) in the Context of Predator-Prey Interactions
The tail flip escape response of the brown shrimp Crangon crangon (L.) in the context of predator-prey interactions Stephen Andrew Arnott This thesis is presented for the degree of Doctor of Philosophy at the University of Glasgow, Institute of Biomedical & Life Sciences, Division of Environmental & Evolutionary Biology. November 1996 ProQuest Number: 11007907 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11007907 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Iq U! S' ^LASCOw' ©NIVERSIfS’ I h b b a r y I Declaration: I declare that this thesis represents, except where a note is made to the contrary, work carried out by myself. The text was composed by myself. Stephen Andrew Amott. November 1996. ACKNOWLEDGEMENTS There are many people who have helped me in all manner of ways during the period of my PhD, and I am extremely grateful for all of the assistance which they have given me. First and foremost, I would like to thank my supervisors Dr. Douglas Neil and Dr. Alan Ansell for offering me the opportunity to undertake this project, for the excellent guidance they have provided through all stages of my laboratory and field work, and especially for their speedy and productive feed-back during my writing up period. -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

Genus Physefocaris, Gen. Nov. Carapace Greatly Inflated
196 Zoologica: Neiv York Zoological Society I" XXV: 11 300 #00 £00 600 700 S00 900 /000 Text-figure 61. Variation in the average carapace length of catches of Para- pandalus richardi made at 100 fathom intervals. Family Physetocaridae, fam. nov. Rostrum present as a broad prolongation of the carapace. First pereio- pods simple. Second pereiopods chelate, with the carpus segmented. No exopods on the third maxillipeds or any of the pereiopods. Terminal joint of the second maxillipeds not applied as a strip to the end of the preceding joint. Mandible without an incisor process or palp. Genus Physefocaris, gen. nov. Carapace greatly inflated. Carpus of second pereiopods consisting of four segments; chela flattened with a very short, broad dactyl. Branchial formula as follows: VII VIII IX x XI XII XIII XIV Podobranchiae ep. ep. ep. ep. ep. ep. Arthrobranchiae Pleurobranchi ae 1 1 1 1 1 Physetocaris microphthalma, sp. nov. Text-figs. 62 and 63. Types: Holotype female (?) Cat. No. 30,523, Department of Tropical Research, New York Zoological Society; Net 798; July 15, 1930; 600 fathoms. 1940] Chace: Bathypelagic, Caridean Crustacea- 197 Physetocaris microphthalma. Holotype. X 6.00. One female (?) ; Net 983, 990, 997, 1003, 1014, 1015, 1016, 1102, 1108, 1115, 1121, 1131, 1137 (?), 1138 (?), 1144, 1149 or 1150; June 2 to August 8, 1931; 500 fathoms. Diagnosis: Carapace with two lateral carinae. Abdomen without any dorsal carinae or spines. Telson deeply sulcate dorsally and broadly truncate at the tip. Eyes very small and set on outside of stalks. Description: Integument extremely thin and fragile. Carapace markedly inflated dorsally and anteriorly to form a very broad, inflated rostrum. -

Identification and Characterization of a Luteinizing Hormone
International Journal of Molecular Sciences Article Identification and Characterization of a Luteinizing Hormone Receptor (LHR) Homolog from the Chinese Mitten Crab Eriocheir sinensis Li-Juan Yuan 1,2,3,4,†, Chao Peng 1,2,3,4,†, Bi-Hai Liu 1,2,3,4,†, Jiang-Bin Feng 1,2,3,4,† and Gao-Feng Qiu 1,2,3,4,*,† 1 National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China; [email protected] (L.-J.Y.); [email protected] (C.P.); [email protected] (B.-H.L.); [email protected] (J.-B.F.) 2 Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China 3 Key Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture, Shanghai Ocean University, Shanghai 201306, China 4 Shanghai Engineering Research Center of Aquaculture, Shanghai Ocean University, Shanghai 201306, China * Correspondence: [email protected]; Tel./Fax: +86-21-61900436 † These authors contributed equally to this work. Received: 6 March 2019; Accepted: 4 April 2019; Published: 8 April 2019 Abstract: Luteinizing hormone (LH), a pituitary gonadotropin, coupled with LH receptor (LHR) is essential for the regulation of the gonadal maturation in vertebrates. Although LH homolog has been detected by immunocytochemical analysis, and its possible role in ovarian maturation was revealed in decapod crustacean, so far there is no molecular evidence for the existence of LHR. In this study, we cloned a novel LHR homolog (named EsLHR) from the Chinese mitten crab Eriocheir sinensis. The complete sequence of the EsLHR cDNA was 2775bp, encoding a protein of 924 amino acids, sharing 71% amino acids identity with the ant Zootermopsis nevadensis LHR. -

Prawn Fauna (Crustacea: Decapoda) of India - an Annotated Checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean Prawns
Available online at: www.mbai.org.in doi: 10.6024/jmbai.2012.54.1.01697-08 Prawn fauna (Crustacea: Decapoda) of India - An annotated checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean prawns E. V. Radhakrishnan*1, V. D. Deshmukh2, G. Maheswarudu3, Jose Josileen 1, A. P. Dineshbabu4, K. K. Philipose5, P. T. Sarada6, S. Lakshmi Pillai1, K. N. Saleela7, Rekhadevi Chakraborty1, Gyanaranjan Dash8, C.K. Sajeev1, P. Thirumilu9, B. Sridhara4, Y Muniyappa4, A.D.Sawant2, Narayan G Vaidya5, R. Dias Johny2, J. B. Verma3, P.K.Baby1, C. Unnikrishnan7, 10 11 11 1 7 N. P. Ramachandran , A. Vairamani , A. Palanichamy , M. Radhakrishnan and B. Raju 1CMFRI HQ, Cochin, 2Mumbai RC of CMFRI, 3Visakhapatnam RC of CMFRI, 4Mangalore RC of CMFRI, 5Karwar RC of CMFRI, 6Tuticorin RC of CMFRI, 7Vizhinjam RC of CMFRI, 8Veraval RC of CMFRI, 9Madras RC of CMFRI, 10Calicut RC of CMFRI, 11Mandapam RC of CMFRI *Correspondence e-mail: [email protected] Received: 07 Sep 2011, Accepted: 15 Mar 2012, Published: 30 Apr 2012 Original Article Abstract Many penaeoid prawns are of considerable value for the fishing Introduction industry and aquaculture operations. The annual estimated average landing of prawns from the fishery in India was 3.98 The prawn fauna inhabiting the marine, estuarine and lakh tonnes (2008-10) of which 60% were contributed by freshwater ecosystems of India are diverse and fairly well penaeid prawns. An additional 1.5 lakh tonnes is produced from known. Significant contributions to systematics of marine aquaculture. During 2010-11, India exported US $ 2.8 billion worth marine products, of which shrimp contributed 3.09% in prawns of Indian region were that of Milne Edwards (1837), volume and 69.5% in value of the total export. -
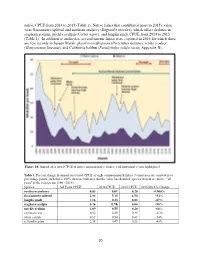
Suisun Marsh Fish Report 2015 Final
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Circadian Clocks in Crustaceans: Identified Neuronal and Cellular Systems
Circadian clocks in crustaceans: identified neuronal and cellular systems Johannes Strauss, Heinrich Dircksen Department of Zoology, Stockholm University, Svante Arrhenius vag 18A, S-10691 Stockholm, Sweden TABLE OF CONTENTS 1. Abstract 2. Introduction: crustacean circadian biology 2.1. Rhythms and circadian phenomena 2.2. Chronobiological systems in Crustacea 2.3. Pacemakers in crustacean circadian systems 3. The cellular basis of crustacean circadian rhythms 3.1. The retina of the eye 3.1.1. Eye pigment migration and its adaptive role 3.1.2. Receptor potential changes of retinular cells in the electroretinogram (ERG) 3.2. Eyestalk systems and mediators of circadian rhythmicity 3.2.1. Red pigment concentrating hormone (RPCH) 3.2.2. Crustacean hyperglycaemic hormone (CHH) 3.2.3. Pigment-dispersing hormone (PDH) 3.2.4. Serotonin 3.2.5. Melatonin 3.2.6. Further factors with possible effects on circadian rhythmicity 3.3. The caudal photoreceptor of the crayfish terminal abdominal ganglion (CPR) 3.4. Extraretinal brain photoreceptors 3.5. Integration of distributed circadian clock systems and rhythms 4. Comparative aspects of crustacean clocks 4.1. Evolution of circadian pacemakers in arthropods 4.2. Putative clock neurons conserved in crustaceans and insects 4.3. Clock genes in crustaceans 4.3.1. Current knowledge about insect clock genes 4.3.2. Crustacean clock-gene 4.3.3. Crustacean period-gene 4.3.4. Crustacean cryptochrome-gene 5. Perspective 6. Acknowledgements 7. References 1. ABSTRACT Circadian rhythms are known for locomotory and reproductive behaviours, and the functioning of sensory organs, nervous structures, metabolism and developmental processes. The mechanisms and cellular bases of control are mainly inferred from circadian phenomenologies, ablation experiments and pharmacological approaches. -

From the Bohol Sea, the Philippines
THE RAFFLES BULLETIN OF ZOOLOGY 2008 RAFFLES BULLETIN OF ZOOLOGY 2008 56(2): 385–404 Date of Publication: 31 Aug.2008 © National University of Singapore NEW GENERA AND SPECIES OF EUXANTHINE CRABS (CRUSTACEA: DECAPODA: BRACHYURA: XANTHIDAE) FROM THE BOHOL SEA, THE PHILIPPINES Jose Christopher E. Mendoza Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore 117543; Institute of Biology, University of the Philippines, Diliman, Quezon City, 1101, Philippines Email: [email protected] Peter K. L. Ng Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore 117543, Republic of Singapore Email: [email protected] ABSTRACT. – Two new genera and four new xanthid crab species belonging to the subfamily Euxanthinae Alcock (Crustacea: Decapoda: Brachyura) are described from the Bohol Sea, central Philippines. Rizalthus, new genus, with just one species, R. anconis, new species, can be distinguished from allied genera by characters of the carapace, epistome, chelipeds, male abdomen and male fi rst gonopod. Visayax, new genus, contains two new species, V. osteodictyon and V. estampadori, and can be distinguished from similar genera using a combination of features of the carapace, epistome, thoracic sternum, male abdomen, pereiopods and male fi rst gonopod. A new species of Hepatoporus Serène, H. pumex, is also described. It is distinguished from congeners by the unique morphology of its front, carapace sculpturing, form of the subhepatic cavity and structure of the male fi rst gonopod. KEY WORDS. – Crustacea, Xanthidae, Euxanthinae, Rizalthus, Visayax, Hepatoporus, Panglao 2004, the Philippines. INTRODUCTION & Jeng, 2006; Anker et al., 2006; Dworschak, 2006; Marin & Chan, 2006; Ahyong & Ng, 2007; Anker & Dworschak, There are currently 24 genera and 83 species in the xanthid 2007; Manuel-Santos & Ng, 2007; Mendoza & Ng, 2007; crab subfamily Euxanthinae worldwide, with most occurring Ng & Castro, 2007; Ng & Manuel-Santos, 2007; Ng & in the Indo-Pacifi c (Ng & McLay, 2007; Ng et al., 2008). -
New Records and Description of Two New Species Of
A peer-reviewed open-access journal ZooKeys 671: 131–153New (2017) records and description of two new species of carideans shrimps... 131 doi: 10.3897/zookeys.671.9081 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research New records and description of two new species of carideans shrimps from Bahía Santa María- La Reforma lagoon, Gulf of California, Mexico (Crustacea, Caridea, Alpheidae and Processidae) José Salgado-Barragán1, Manuel Ayón-Parente2, Pilar Zamora-Tavares2 1 Laboratorio de Invertebrados Bentónicos, Unidad Académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, México 2 Centro Universitario de Ciencias Agrope- cuarias, Universidad de Guadalajara, México Corresponding author: José Salgado-Barragán ([email protected]) Academic editor: S. De Grave | Received 4 May 2016 | Accepted 22 March 2017 | Published 27 April 2017 http://zoobank.org/9742DC49-F925-4B4B-B440-17354BDDB4B5 Citation: Salgado-Barragán J, Ayón-Parente M, Zamora-Tavares P (2017) New records and description of two new species of carideans shrimps from Bahía Santa María-La Reforma lagoon, Gulf of California, Mexico (Crustacea, Caridea, Alpheidae and Processidae). ZooKeys 671: 131–153. https://doi.org/10.3897/zookeys.671.9081 Abstract Two new species of the family Alpheidae: Alpheus margaritae sp. n. and Leptalpheus melendezensis sp. n. are described from Santa María-La Reforma, coastal lagoon, SE Gulf of California. Alpheus margaritae sp. n. is closely related to A. antepaenultimus and A. mazatlanicus from the Eastern Pacific and to A. chacei from the Western Atlantic, but can be differentiated from these by a combination of characters, especially the morphology of the scaphocerite and the first pereopods.