An Overview of the Decapoda with Glossary and References
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Manager: Kin Tsoi Chef: Chun Wing Lee Champagne Glass Bottle
Authentic Hong Kong style cuisine Manager: Kin Tsoi Chef: Chun Wing Lee champagne glass bottle 104 nicolase feuillatte, brut, france 187ml 26 100 moet et chandon, brut imperial, france 375ml 67 101 veuve cliquot, yellow label, france 375ml 69 1000 moet et chandon, brut imperial, france 97 1002 veuve cliquot, yellow label, france 119 sparkling wines 105 tiamo, prosecco, italy 187ml 9 1203 domaine ste. michelle, brut, washington 27 1210 montsarra, cava drut, spain 37 white wines 201 tally, chardonnay, california 375ml 33 2019 milbrandt vineyards, chardonnay traditions,washington 9 32 2901 santa julia, chardonnay, organica, argentina 28 2609 lageder, pinot grigio “riff” italy 9 32 2908 lawson’s, sauvignon blanc, new zealand 29 2308 ferrari-carano, fume blanc, california 10 34 2501 heinz eifel, riesling, germany 9 32 2608 degiorgis, moscato d’ asti, italy 34 2316 mountain view, white zinfandel, california 9 32 red wines glass bottle 407 alexander valley, cabernet sauvignon, california 375ml 21 3000 alexander valley, cabernet sauvignon, california 32 3006 dante, cabernet sauvignon, california 9 32 3056 conn creek, herrick red, california 31 3501 cartlidge & brown, merlot, california 9 32 3503 tortoise creek, merlot, california 9 29 3600 a to z, pinot noir, oregon 12 44 4039 tortoise creek “le charmes”, pinot noir, france 10 34 3705 seghesio, zinfandel, california 55 4620 conquista, malbec, argentina 25 sake cup carafe sake cup carafe 10.50 30 hana fuji apple 9.50 27 tozai typhoon hana lychee 9.50 27 tozai living jewel 11.50 33 hana peach 9.50 -

Wild Species 2010 the GENERAL STATUS of SPECIES in CANADA
Wild Species 2010 THE GENERAL STATUS OF SPECIES IN CANADA Canadian Endangered Species Conservation Council National General Status Working Group This report is a product from the collaboration of all provincial and territorial governments in Canada, and of the federal government. Canadian Endangered Species Conservation Council (CESCC). 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group: 302 pp. Available in French under title: Espèces sauvages 2010: La situation générale des espèces au Canada. ii Abstract Wild Species 2010 is the third report of the series after 2000 and 2005. The aim of the Wild Species series is to provide an overview on which species occur in Canada, in which provinces, territories or ocean regions they occur, and what is their status. Each species assessed in this report received a rank among the following categories: Extinct (0.2), Extirpated (0.1), At Risk (1), May Be At Risk (2), Sensitive (3), Secure (4), Undetermined (5), Not Assessed (6), Exotic (7) or Accidental (8). In the 2010 report, 11 950 species were assessed. Many taxonomic groups that were first assessed in the previous Wild Species reports were reassessed, such as vascular plants, freshwater mussels, odonates, butterflies, crayfishes, amphibians, reptiles, birds and mammals. Other taxonomic groups are assessed for the first time in the Wild Species 2010 report, namely lichens, mosses, spiders, predaceous diving beetles, ground beetles (including the reassessment of tiger beetles), lady beetles, bumblebees, black flies, horse flies, mosquitoes, and some selected macromoths. The overall results of this report show that the majority of Canada’s wild species are ranked Secure. -

Carcinization in the Anomura–Fact Or Fiction? II. Evidence from Larval
Contributions to Zoology, 73 (3) 165-205 (2004) SPB Academic Publishing bv, The Hague Carcinization in the Anomura - fact or fiction? II. Evidence from larval, megalopal and early juvenile morphology Patsy+A. McLaughlin Rafael Lemaitre² & Christopher+C. Tudge² ¹, 1 Shannon Point Marine Center, Western Washington University, 1900 Shannon Point Road, Anacortes, 2 Washington 98221-908IB, U.S.A; Department ofSystematic Biology, NationalMuseum ofNatural History, Smithsonian Institution, P.O. Box 37012, Washington, D.C. 20013-7012, U.S.A. Keywords: Carcinization, Anomura, Paguroidea, Lithodidae, Paguridae, Lomisidae, Porcellanidae, larval, megalopal and early juvenile morphology, pleonal tergites Abstract Existing hypotheses 169 Developmental data 170 Results 177 In this second carcinization in the Anomura ofa two-part series, From hermit to king, or king to hermit? 179 has been reviewed from early juvenile, megalopal, and larval Analysis by Richter & Scholtz 179 perspectives. Data from megalopal and early juvenile develop- Questions of asymmetry- 180 ment in ten ofthe Lithodidae have genera provided unequivo- Pleopod loss and gain 18! cal evidence that earlier hypotheses regarding evolution ofthe Uropod loss and transformation 182 king crab erroneous. of and pleon were A pattern sundering, - Polarity or what constitutes a primitive character decalcification has been traced from the megalopal stage through state? 182 several early crabs stages in species ofLithodes and Paralomis, Semaphoronts 184 with evidence from in other supplemental species eight genera. Megalopa/early juvenile characters and character Of major significance has been the attention directed to the states 185 inmarginallithodidsplatesareofnotthehomologoussecond pleomere,with thewhichadult whenso-calledseparated“mar- Cladistic analyses 189 Lomisoidea 192 ginal plates” ofthe three megalopal following tergites. -

Report of the Working Group on the Biology and Life History of Crabs (WGCRAB)
ICES WGCRAB REPORT 2012 SCICOM STEERING GROUP ON ECOSYSTEM FUNCTIONS ICES CM 2012/SSGEF:08 REF. SSGEF, SCICOM, ACOM Report of the Working Group on the Biology and Life History of Crabs (WGCRAB) 14–18 May 2012 Port Erin, Isle of Man, UK International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2012. Report of the Working Group on the Biology and Life History of Crabs (WGCRAB), 14–18 May 2012. ICES CM 2012/SSGEF:08 80pp. For permission to reproduce material from this publication, please apply to the Gen- eral Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2012 International Council for the Exploration of the Sea ICES WGCRAB Report 2012 | i Contents Executive summary ................................................................................................................ 1 1 Introduction .................................................................................................................... 2 2 Adoption of the agenda ................................................................................................ 2 3 Terms of reference 2011 ................................................................................................ 2 4 -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

Lobsters and Crabs As Potential Vectors for Tunicate Dispersal in the Southern Gulf of St. Lawrence, Canada
Aquatic Invasions (2009) Volume 4, Issue 1: 105-110 This is an Open Access article; doi: 10.3391/ai. 2009.4.1.11 © 2009 The Author(s). Journal compilation © 2009 REABIC Special issue “Proceedings of the 2nd International Invasive Sea Squirt Conference” (October 2-4, 2007, Prince Edward Island, Canada) Andrea Locke and Mary Carman (Guest Editors) Research article Lobsters and crabs as potential vectors for tunicate dispersal in the southern Gulf of St. Lawrence, Canada Renée Y. Bernier, Andrea Locke* and John Mark Hanson Fisheries and Oceans Canada, Gulf Fisheries Centre, P.O. Box 5030, Moncton, NB, E1C 9B6 Canada * Corresponding author E-mail: [email protected] Received 20 February 2008; accepted for special issue 5 June 2008; accepted in revised form 22 December 2008; published online 16 January 2009 Abstract Following anecdotal reports of tunicates on the carapaces of rock crab (Cancer irroratus) and American lobster (Homarus americanus), we evaluated the role of these species and northern lady crab Ovalipes ocellatus as natural vectors for the spread of invasive tunicates in the southern Gulf of St. Lawrence. Several hundred adult specimens of crabs and lobster from two tunicate- infested estuaries and Northumberland Strait were examined for epibionts. Small patches of Botrylloides violaceus were found on rock crabs examined from Savage Harbour and a small colony of Botryllus schlosseri was found on one lobster from St. Peters Bay. Lobster and lady crab collected in Northumberland Strait had no attached colonial tunicates but small sea grapes (Molgula sp.) were found attached on the underside of 5.5% of the rock crab and on 2.5% of lobster collected in Northumberland Strait in August 2006. -

Balanus Trigonus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Settlement of the barnacle Balanus trigonus BRAZILIAN CRUSTACEAN SOCIETY Darwin, 1854, on Panulirus gracilis Streets, 1871, in western Mexico e-ISSN 2358-2936 www.scielo.br/nau 1 orcid.org/0000-0001-9187-6080 www.crustacea.org.br Michel E. Hendrickx Evlin Ramírez-Félix2 orcid.org/0000-0002-5136-5283 1 Unidad académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. A.P. 811, Mazatlán, Sinaloa, 82000, Mexico 2 Oficina de INAPESCA Mazatlán, Instituto Nacional de Pesca y Acuacultura. Sábalo- Cerritos s/n., Col. Estero El Yugo, Mazatlán, 82112, Sinaloa, Mexico. ZOOBANK http://zoobank.org/urn:lsid:zoobank.org:pub:74B93F4F-0E5E-4D69- A7F5-5F423DA3762E ABSTRACT A large number of specimens (2765) of the acorn barnacle Balanus trigonus Darwin, 1854, were observed on the spiny lobster Panulirus gracilis Streets, 1871, in western Mexico, including recently settled cypris (1019 individuals or 37%) and encrusted specimens (1746) of different sizes: <1.99 mm, 88%; 1.99 to 2.82 mm, 8%; >2.82 mm, 4%). Cypris settled predominantly on the carapace (67%), mostly on the gastric area (40%), on the left or right orbital areas (35%), on the head appendages, and on the pereiopods 1–3. Encrusting individuals were mostly small (84%); medium-sized specimens accounted for 11% and large for 5%. On the cephalothorax, most were observed in branchial (661) and orbital areas (240). Only 40–41 individuals were found on gastric and cardiac areas. Some individuals (246), mostly small (95%), were observed on the dorsal portion of somites. -
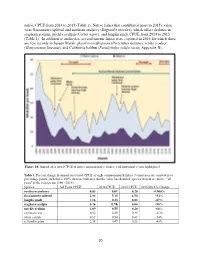
Suisun Marsh Fish Report 2015 Final
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Circadian Clocks in Crustaceans: Identified Neuronal and Cellular Systems
Circadian clocks in crustaceans: identified neuronal and cellular systems Johannes Strauss, Heinrich Dircksen Department of Zoology, Stockholm University, Svante Arrhenius vag 18A, S-10691 Stockholm, Sweden TABLE OF CONTENTS 1. Abstract 2. Introduction: crustacean circadian biology 2.1. Rhythms and circadian phenomena 2.2. Chronobiological systems in Crustacea 2.3. Pacemakers in crustacean circadian systems 3. The cellular basis of crustacean circadian rhythms 3.1. The retina of the eye 3.1.1. Eye pigment migration and its adaptive role 3.1.2. Receptor potential changes of retinular cells in the electroretinogram (ERG) 3.2. Eyestalk systems and mediators of circadian rhythmicity 3.2.1. Red pigment concentrating hormone (RPCH) 3.2.2. Crustacean hyperglycaemic hormone (CHH) 3.2.3. Pigment-dispersing hormone (PDH) 3.2.4. Serotonin 3.2.5. Melatonin 3.2.6. Further factors with possible effects on circadian rhythmicity 3.3. The caudal photoreceptor of the crayfish terminal abdominal ganglion (CPR) 3.4. Extraretinal brain photoreceptors 3.5. Integration of distributed circadian clock systems and rhythms 4. Comparative aspects of crustacean clocks 4.1. Evolution of circadian pacemakers in arthropods 4.2. Putative clock neurons conserved in crustaceans and insects 4.3. Clock genes in crustaceans 4.3.1. Current knowledge about insect clock genes 4.3.2. Crustacean clock-gene 4.3.3. Crustacean period-gene 4.3.4. Crustacean cryptochrome-gene 5. Perspective 6. Acknowledgements 7. References 1. ABSTRACT Circadian rhythms are known for locomotory and reproductive behaviours, and the functioning of sensory organs, nervous structures, metabolism and developmental processes. The mechanisms and cellular bases of control are mainly inferred from circadian phenomenologies, ablation experiments and pharmacological approaches. -

How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 25 December 2020 doi:10.20944/preprints202012.0664.v1 How to become a crab: Phenotypic constraints on a recurring body plan Joanna M. Wolfe1*, Javier Luque1,2,3, Heather D. Bracken-Grissom4 1 Museum of Comparative Zoology and Department of Organismic & Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2 Smithsonian Tropical Research Institute, Balboa–Ancon, 0843–03092, Panama, Panama 3 Department of Earth and Planetary Sciences, Yale University, New Haven, CT 06520-8109, USA 4 Institute of Environment and Department of Biological Sciences, Florida International University, Biscayne Bay Campus, 3000 NE 151 Street, North Miami, FL 33181, USA * E-mail: [email protected] Summary: A fundamental question in biology is whether phenotypes can be predicted by ecological or genomic rules. For over 140 years, convergent evolution of the crab-like body plan (with a wide and flattened shape, and a bent abdomen) at least five times in decapod crustaceans has been known as ‘carcinization’. The repeated loss of this body plan has been identified as ‘decarcinization’. We offer phylogenetic strategies to include poorly known groups, and direct evidence from fossils, that will resolve the pattern of crab evolution and the degree of phenotypic variation within crabs. Proposed ecological advantages of the crab body are summarized into a hypothesis of phenotypic integration suggesting correlated evolution of the carapace shape and abdomen. Our premise provides fertile ground for future studies of the genomic and developmental basis, and the predictability, of the crab-like body form. Keywords: Crustacea, Anomura, Brachyura, Carcinization, Phylogeny, Convergent evolution, Morphological integration 1 © 2020 by the author(s).