Thermodynamic Equations Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

Thermodynamics and Phase Diagrams
Thermodynamics and Phase Diagrams Arthur D. Pelton Centre de Recherche en Calcul Thermodynamique (CRCT) École Polytechnique de Montréal C.P. 6079, succursale Centre-ville Montréal QC Canada H3C 3A7 Tél : 1-514-340-4711 (ext. 4531) Fax : 1-514-340-5840 E-mail : [email protected] (revised Aug. 10, 2011) 2 1 Introduction An understanding of phase diagrams is fundamental and essential to the study of materials science, and an understanding of thermodynamics is fundamental to an understanding of phase diagrams. A knowledge of the equilibrium state of a system under a given set of conditions is the starting point in the description of many phenomena and processes. A phase diagram is a graphical representation of the values of the thermodynamic variables when equilibrium is established among the phases of a system. Materials scientists are most familiar with phase diagrams which involve temperature, T, and composition as variables. Examples are T-composition phase diagrams for binary systems such as Fig. 1 for the Fe-Mo system, isothermal phase diagram sections of ternary systems such as Fig. 2 for the Zn-Mg-Al system, and isoplethal (constant composition) sections of ternary and higher-order systems such as Fig. 3a and Fig. 4. However, many useful phase diagrams can be drawn which involve variables other than T and composition. The diagram in Fig. 5 shows the phases present at equilibrium o in the Fe-Ni-O2 system at 1200 C as the equilibrium oxygen partial pressure (i.e. chemical potential) is varied. The x-axis of this diagram is the overall molar metal ratio in the system. -
Mol of Solute Molality ( ) = Kg Solvent M
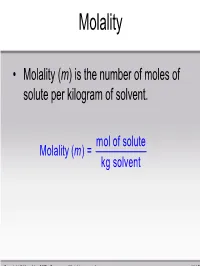
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant. -

Comparative Examination of Nonequilibrium Thermodynamic Models of Thermodiffusion in Liquids †
Proceedings Comparative Examination of Nonequilibrium Thermodynamic Models of Thermodiffusion in Liquids † Semen N. Semenov 1,* and Martin E. Schimpf 2 1 Institute of Biochemical Physics RAS, Moscow 119334, Russia 2 Department of Chemistry, Boise State University, Boise, ID 83725, USA; [email protected] * Correspondence: [email protected] † Presented at the 5th International Electronic Conference on Entropy and Its Applications, 18–30 November 2019; Available online: https://ecea-5.sciforum.net/. Published: 17 November 2019 Abstract: We analyze existing models for material transport in non-isothermal non-electrolyte liquid mixtures that utilize non-equilibrium thermodynamics. Many different sets of equations for material have been derived that, while based on the same fundamental expression of entropy production, utilize different terms of the temperature- and concentration-induced gradients in the chemical potential to express the material flux. We reason that only by establishing a system of transport equations that satisfies the following three requirements can we obtain a valid thermodynamic model of thermodiffusion based on entropy production, and understand the underlying physical mechanism: (1) Maintenance of mechanical equilibrium in a closed steady-state system, expressed by a form of the Gibbs–Duhem equation that accounts for all the relevant gradients in concentration, temperature, and pressure and respective thermodynamic forces; (2) thermodiffusion (thermophoresis) is zero in pure unbounded liquids (i.e., in the absence of wall effects); (3) invariance in the derived concentrations of components in a mixture, regardless of which concentration or material flux is considered to be the dependent versus independent variable in an overdetermined system of material transport equations. The analysis shows that thermodiffusion in liquids is based on the entropic mechanism. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Equation of State - Wikipedia, the Free Encyclopedia 頁 1 / 8
Equation of state - Wikipedia, the free encyclopedia 頁 1 / 8 Equation of state From Wikipedia, the free encyclopedia In physics and thermodynamics, an equation of state is a relation between state variables.[1] More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure, volume, or internal energy. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids, and even the interior of stars. Thermodynamics Contents ■ 1 Overview ■ 2Historical ■ 2.1 Boyle's law (1662) ■ 2.2 Charles's law or Law of Charles and Gay-Lussac (1787) Branches ■ 2.3 Dalton's law of partial pressures (1801) ■ 2.4 The ideal gas law (1834) Classical · Statistical · Chemical ■ 2.5 Van der Waals equation of state (1873) Equilibrium / Non-equilibrium ■ 3 Major equations of state Thermofluids ■ 3.1 Classical ideal gas law Laws ■ 4 Cubic equations of state Zeroth · First · Second · Third ■ 4.1 Van der Waals equation of state ■ 4.2 Redlich–Kwong equation of state Systems ■ 4.3 Soave modification of Redlich-Kwong State: ■ 4.4 Peng–Robinson equation of state Equation of state ■ 4.5 Peng-Robinson-Stryjek-Vera equations of state Ideal gas · Real gas ■ 4.5.1 PRSV1 Phase of matter · Equilibrium ■ 4.5.2 PRSV2 Control volume · Instruments ■ 4.6 Elliott, Suresh, Donohue equation of state Processes: -

Chemical Engineering Thermodynamics
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY | 1 TABLE OF CONTENTS Symbol Dictionary ........................................................................................................................ 3 1. Measured Thermodynamic Properties and Other Basic Concepts .................................. 5 1.1 Preliminary Concepts – The Language of Thermodynamics ........................................................ 5 1.2 Measured Thermodynamic Properties .......................................................................................... 5 1.2.1 Volume .................................................................................................................................................... 5 1.2.2 Temperature ............................................................................................................................................. 5 1.2.3 Pressure .................................................................................................................................................... 6 1.3 Equilibrium ................................................................................................................................... 7 1.3.1 Fundamental Definitions .......................................................................................................................... 7 1.3.2 Independent and Dependent Thermodynamic Properties ........................................................................ 7 1.3.3 Phases ..................................................................................................................................................... -

Molal Freezing Point Depression Constants SCIENTIFIC Calculating Kf Values
Molal Freezing Point Depression Constants SCIENTIFIC Calculating Kf Values Introduction The freezing point of a liquid is the temperature at which the forces of attraction among molecules are just enough to cause a phase change from the liquid state to the solid state. It is the temperature at which the liquid and solid phases exist in equilib- rium. The solvent molecules in a solution are somewhat more separated from each other than they are in a pure solvent due to the interference of solute particles. Therefore, the temperature of a solution must be lowered below the freezing point of the pure solvent in order to freeze it. The freezing point depression (∆Tf) of solutions of nonelectrolytes has been found to be equal to the molality (m) of the solute times a proportionality constant called the molal freezing point depression constant (Kf). Therefore, ∆Tf = Kf × m, Equation 1 where m is the number of moles of solute per kilograms of solvent. Concepts • Freezing point depression • Colligative properties Discussion The determination of the molecular mass of a substance from the freezing point depression is included in many high school and most college laboratory manuals. In a typical experiment, the freezing point of the pure solvent is measured followed by the freezing point of a solution of known molality. Dividing the freezing point depression (∆Tf) by the solution molality (m) gives the molal freezing point constant (Kf) of the solvent, according to Equation 1. The second part of the lab consists of using the Kf value determined in part one to determine the molality, and ultimately the molecular mass of an unknown substance. -

Definition of the Ideal Gas
ON THE DEFINITION OF THE IDEAL GAS. By Edgar Bucidngham. I . Nature and purpose of the definition.—The notion of the ideal gas is that of a gas having particularly simple physical properties to which the properties of the real gases may be considered as approximations; or of a standard to which the real gases may be referred, the properties of the ideal gas being simply defined and the properties of the real gases being then expressible as the prop- erties of the standard plus certain corrections which pertain to the individual gases. The smaller these corrections the more nearly the real gas approaches to being in the "ideal state." This con- ception grew naturally from the fact that the earlier experiments on gases showed that they did not differ much in their physical properties, so that it was possible to define an ideal standard in such a way that the corrections above referred to should in fact all be ''small" in terms of the unavoidable errors of experiment. Such a conception would hardly arise to-day, or if it did, would not be so simple as that which has come down to us from earlier times when the art of experimenting upon gases was less advanced. It is evident that a quantitative definition of the ideal standard gas needs to be more or less complete and precise according to the nature of the problem under immediate consideration. If changes of temperature and of internal energy play no part, all that is usually needed is a standard relation between pressure and volume.