Maxwell Relations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamic Potentials and Natural Variables
Revista Brasileira de Ensino de Física, vol. 42, e20190127 (2020) Articles www.scielo.br/rbef cb DOI: http://dx.doi.org/10.1590/1806-9126-RBEF-2019-0127 Licença Creative Commons Thermodynamic Potentials and Natural Variables M. Amaku1,2, F. A. B. Coutinho*1, L. N. Oliveira3 1Universidade de São Paulo, Faculdade de Medicina, São Paulo, SP, Brasil 2Universidade de São Paulo, Faculdade de Medicina Veterinária e Zootecnia, São Paulo, SP, Brasil 3Universidade de São Paulo, Instituto de Física de São Carlos, São Carlos, SP, Brasil Received on May 30, 2019. Revised on September 13, 2018. Accepted on October 4, 2019. Most books on Thermodynamics explain what thermodynamic potentials are and how conveniently they describe the properties of physical systems. Certain books add that, to be useful, the thermodynamic potentials must be expressed in their “natural variables”. Here we show that, given a set of physical variables, an appropriate thermodynamic potential can always be defined, which contains all the thermodynamic information about the system. We adopt various perspectives to discuss this point, which to the best of our knowledge has not been clearly presented in the literature. Keywords: Thermodynamic Potentials, natural variables, Legendre transforms. 1. Introduction same statement cannot be applied to the temperature. In real fluids, even in simple ones, the proportionality Basic concepts are most easily understood when we dis- to T is washed out, and the Internal Energy is more cuss simple systems. Consider an ideal gas in a cylinder. conveniently expressed as a function of the entropy and The cylinder is closed, its walls are conducting, and a volume: U = U(S, V ). -

PDF Version of Helmholtz Free Energy
Free energy Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Recall that dS ≥ δq/T so we have dU ≤ TdS Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Recall that dS ≥ δq/T so we have dU ≤ TdS which leads to dU - TdS ≤ 0 Since T and V are constant we can write this as d(U - TS) ≤ 0 The quantity in parentheses is a measure of the spontaneity of the system that depends on known state functions. Definition of Helmholtz Free Energy We define a new state function: A = U -TS such that dA ≤ 0. We call A the Helmholtz free energy. At constant T and V the Helmholtz free energy will decrease until all possible spontaneous processes have occurred. At that point the system will be in equilibrium. The condition for equilibrium is dA = 0. A time Definition of Helmholtz Free Energy Expressing the change in the Helmholtz free energy we have ∆A = ∆U – T∆S for an isothermal change from one state to another. The condition for spontaneous change is that ∆A is less than zero and the condition for equilibrium is that ∆A = 0. We write ∆A = ∆U – T∆S ≤ 0 (at constant T and V) Definition of Helmholtz Free Energy Expressing the change in the Helmholtz free energy we have ∆A = ∆U – T∆S for an isothermal change from one state to another. -

Chapter 3. Second and Third Law of Thermodynamics
Chapter 3. Second and third law of thermodynamics Important Concepts Review Entropy; Gibbs Free Energy • Entropy (S) – definitions Law of Corresponding States (ch 1 notes) • Entropy changes in reversible and Reduced pressure, temperatures, volumes irreversible processes • Entropy of mixing of ideal gases • 2nd law of thermodynamics • 3rd law of thermodynamics Math • Free energy Numerical integration by computer • Maxwell relations (Trapezoidal integration • Dependence of free energy on P, V, T https://en.wikipedia.org/wiki/Trapezoidal_rule) • Thermodynamic functions of mixtures Properties of partial differential equations • Partial molar quantities and chemical Rules for inequalities potential Major Concept Review • Adiabats vs. isotherms p1V1 p2V2 • Sign convention for work and heat w done on c=C /R vm system, q supplied to system : + p1V1 p2V2 =Cp/CV w done by system, q removed from system : c c V1T1 V2T2 - • Joule-Thomson expansion (DH=0); • State variables depend on final & initial state; not Joule-Thomson coefficient, inversion path. temperature • Reversible change occurs in series of equilibrium V states T TT V P p • Adiabatic q = 0; Isothermal DT = 0 H CP • Equations of state for enthalpy, H and internal • Formation reaction; enthalpies of energy, U reaction, Hess’s Law; other changes D rxn H iD f Hi i T D rxn H Drxn Href DrxnCpdT Tref • Calorimetry Spontaneous and Nonspontaneous Changes First Law: when one form of energy is converted to another, the total energy in universe is conserved. • Does not give any other restriction on a process • But many processes have a natural direction Examples • gas expands into a vacuum; not the reverse • can burn paper; can't unburn paper • heat never flows spontaneously from cold to hot These changes are called nonspontaneous changes. -

( ∂U ∂T ) = ∂CV ∂V = 0, Which Shows That CV Is Independent of V . 4
so we have ∂ ∂U ∂ ∂U ∂C =0 = = V =0, ∂T ∂V ⇒ ∂V ∂T ∂V which shows that CV is independent of V . 4. Using Maxwell’s relations. Show that (∂H/∂p) = V T (∂V/∂T ) . T − p Start with dH = TdS+ Vdp. Now divide by dp, holding T constant: dH ∂H ∂S [at constant T ]= = T + V. dp ∂p ∂p T T Use the Maxwell relation (Table 9.1 of the text), ∂S ∂V = ∂p − ∂T T p to get the result ∂H ∂V = T + V. ∂p − ∂T T p 97 5. Pressure dependence of the heat capacity. (a) Show that, in general, for quasi-static processes, ∂C ∂2V p = T . ∂p − ∂T2 T p (b) Based on (a), show that (∂Cp/∂p)T = 0 for an ideal gas. (a) Begin with the definition of the heat capacity, δq dS C = = T , p dT dT for a quasi-static process. Take the derivative: ∂C ∂2S ∂2S p = T = T (1) ∂p ∂p∂T ∂T∂p T since S is a state function. Substitute the Maxwell relation ∂S ∂V = ∂p − ∂T T p into Equation (1) to get ∂C ∂2V p = T . ∂p − ∂T2 T p (b) For an ideal gas, V (T )=NkT/p,so ∂V Nk = , ∂T p p ∂2V =0, ∂T2 p and therefore, from part (a), ∂C p =0. ∂p T 98 (a) dU = TdS+ PdV + μdN + Fdx, dG = SdT + VdP+ Fdx, − ∂G F = , ∂x T,P 1 2 G(x)= (aT + b)xdx= 2 (aT + b)x . ∂S ∂F (b) = , ∂x − ∂T T,P x,P ∂S ∂F (c) = = ax, ∂x − ∂T − T,P x,P S(x)= ax dx = 1 ax2. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

7 Apr 2021 Thermodynamic Response Functions . L03–1 Review Of
7 apr 2021 thermodynamic response functions . L03{1 Review of Thermodynamics. 3: Second-Order Quantities and Relationships Maxwell Relations • Idea: Each thermodynamic potential gives rise to several identities among its second derivatives, known as Maxwell relations, which express the integrability of the fundamental identity of thermodynamics for that potential, or equivalently the fact that the potential really is a thermodynamical state function. • Example: From the fundamental identity of thermodynamics written in terms of the Helmholtz free energy, dF = −S dT − p dV + :::, the fact that dF really is the differential of a state function implies that @ @F @ @F @S @p = ; or = : @V @T @T @V @V T;N @T V;N • Other Maxwell relations: From the same identity, if F = F (T; V; N) we get two more relations. Other potentials and/or pairs of variables can be used to obtain additional relations. For example, from the Gibbs free energy and the identity dG = −S dT + V dp + µ dN, we get three relations, including @ @G @ @G @S @V = ; or = − : @p @T @T @p @p T;N @T p;N • Applications: Some measurable quantities, response functions such as heat capacities and compressibilities, are second-order thermodynamical quantities (i.e., their definitions contain derivatives up to second order of thermodynamic potentials), and the Maxwell relations provide useful equations among them. Heat Capacities • Definitions: A heat capacity is a response function expressing how much a system's temperature changes when heat is transferred to it, or equivalently how much δQ is needed to obtain a given dT . The general definition is C = δQ=dT , where for any reversible transformation δQ = T dS, but the value of this quantity depends on the details of the transformation. -

Thermodynamic Analysis Based on the Second-Order Variations of Thermodynamic Potentials
Theoret. Appl. Mech., Vol.35, No.1-3, pp. 215{234, Belgrade 2008 Thermodynamic analysis based on the second-order variations of thermodynamic potentials Vlado A. Lubarda ¤ Abstract An analysis of the Gibbs conditions of stable thermodynamic equi- librium, based on the constrained minimization of the four fundamen- tal thermodynamic potentials, is presented with a particular attention given to the previously unexplored connections between the second- order variations of thermodynamic potentials. These connections are used to establish the convexity properties of all potentials in relation to each other, which systematically deliver thermodynamic relationships between the speci¯c heats, and the isentropic and isothermal bulk mod- uli and compressibilities. The comparison with the classical derivation is then given. Keywords: Gibbs conditions, internal energy, second-order variations, speci¯c heats, thermodynamic potentials 1 Introduction The Gibbs conditions of thermodynamic equilibrium are of great importance in the analysis of the equilibrium and stability of homogeneous and heterogeneous thermodynamic systems [1]. The system is in a thermodynamic equilibrium if its state variables do not spontaneously change with time. As a consequence of the second law of thermodynamics, the equilibrium state of an isolated sys- tem at constant volume and internal energy is the state with the maximum value of the total entropy. Alternatively, among all neighboring states with the ¤Department of Mechanical and Aerospace Engineering, University of California, San Diego; La Jolla, CA 92093-0411, USA and Montenegrin Academy of Sciences and Arts, Rista Stijovi¶ca5, 81000 Podgorica, Montenegro, e-mail: [email protected]; [email protected] 215 216 Vlado A. Lubarda same volume and total entropy, the equilibrium state is one with the lowest total internal energy. -
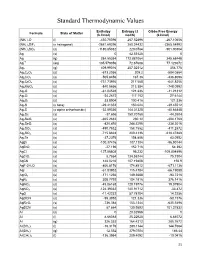
Standard Thermodynamic Values
Standard Thermodynamic Values Enthalpy Entropy (J Gibbs Free Energy Formula State of Matter (kJ/mol) mol/K) (kJ/mol) (NH4)2O (l) -430.70096 267.52496 -267.10656 (NH4)2SiF6 (s hexagonal) -2681.69296 280.24432 -2365.54992 (NH4)2SO4 (s) -1180.85032 220.0784 -901.90304 Ag (s) 0 42.55128 0 Ag (g) 284.55384 172.887064 245.68448 Ag+1 (aq) 105.579056 72.67608 77.123672 Ag2 (g) 409.99016 257.02312 358.778 Ag2C2O4 (s) -673.2056 209.2 -584.0864 Ag2CO3 (s) -505.8456 167.36 -436.8096 Ag2CrO4 (s) -731.73976 217.568 -641.8256 Ag2MoO4 (s) -840.5656 213.384 -748.0992 Ag2O (s) -31.04528 121.336 -11.21312 Ag2O2 (s) -24.2672 117.152 27.6144 Ag2O3 (s) 33.8904 100.416 121.336 Ag2S (s beta) -29.41352 150.624 -39.45512 Ag2S (s alpha orthorhombic) -32.59336 144.01328 -40.66848 Ag2Se (s) -37.656 150.70768 -44.3504 Ag2SeO3 (s) -365.2632 230.12 -304.1768 Ag2SeO4 (s) -420.492 248.5296 -334.3016 Ag2SO3 (s) -490.7832 158.1552 -411.2872 Ag2SO4 (s) -715.8824 200.4136 -618.47888 Ag2Te (s) -37.2376 154.808 43.0952 AgBr (s) -100.37416 107.1104 -96.90144 AgBrO3 (s) -27.196 152.716 54.392 AgCl (s) -127.06808 96.232 -109.804896 AgClO2 (s) 8.7864 134.55744 75.7304 AgCN (s) 146.0216 107.19408 156.9 AgF•2H2O (s) -800.8176 174.8912 -671.1136 AgI (s) -61.83952 115.4784 -66.19088 AgIO3 (s) -171.1256 149.3688 -93.7216 AgN3 (s) 308.7792 104.1816 376.1416 AgNO2 (s) -45.06168 128.19776 19.07904 AgNO3 (s) -124.39032 140.91712 -33.472 AgO (s) -11.42232 57.78104 14.2256 AgOCN (s) -95.3952 121.336 -58.1576 AgReO4 (s) -736.384 153.1344 -635.5496 AgSCN (s) 87.864 130.9592 101.37832 Al (s) -

Thermodynamics and Phase Diagrams
Thermodynamics and Phase Diagrams Arthur D. Pelton Centre de Recherche en Calcul Thermodynamique (CRCT) École Polytechnique de Montréal C.P. 6079, succursale Centre-ville Montréal QC Canada H3C 3A7 Tél : 1-514-340-4711 (ext. 4531) Fax : 1-514-340-5840 E-mail : [email protected] (revised Aug. 10, 2011) 2 1 Introduction An understanding of phase diagrams is fundamental and essential to the study of materials science, and an understanding of thermodynamics is fundamental to an understanding of phase diagrams. A knowledge of the equilibrium state of a system under a given set of conditions is the starting point in the description of many phenomena and processes. A phase diagram is a graphical representation of the values of the thermodynamic variables when equilibrium is established among the phases of a system. Materials scientists are most familiar with phase diagrams which involve temperature, T, and composition as variables. Examples are T-composition phase diagrams for binary systems such as Fig. 1 for the Fe-Mo system, isothermal phase diagram sections of ternary systems such as Fig. 2 for the Zn-Mg-Al system, and isoplethal (constant composition) sections of ternary and higher-order systems such as Fig. 3a and Fig. 4. However, many useful phase diagrams can be drawn which involve variables other than T and composition. The diagram in Fig. 5 shows the phases present at equilibrium o in the Fe-Ni-O2 system at 1200 C as the equilibrium oxygen partial pressure (i.e. chemical potential) is varied. The x-axis of this diagram is the overall molar metal ratio in the system. -

Thermodynamics in Earth and Planetary Sciences
Thermodynamics in Earth and Planetary Sciences Jibamitra Ganguly Thermodynamics in Earth and Planetary Sciences 123 Prof. Jibamitra Ganguly University of Arizona Dept. Geosciences 1040 E. Fourth St. Tucson AZ 85721-0077 Gould Simpson Bldg. USA [email protected] ISBN: 978-3-540-77305-4 e-ISBN: 978-3-540-77306-1 DOI 10.1007/978-3-540-77306-1 Library of Congress Control Number: 2008930074 c 2008 Springer-Verlag Berlin Heidelberg This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: deblik, Berlin Printed on acid-free paper 987654321 springer.com A theory is the more impressive the greater the simplicity of its premises, the more different kind of things it relates, and the more extended its area of applicability. Therefore the deep impression that classical thermodynamics made upon me. It is the only physical theory of universal content which I am convinced will never be overthrown, within the framework of applicability of its basic concepts. -

Energy and Enthalpy Thermodynamics
Energy and Energy and Enthalpy Thermodynamics The internal energy (E) of a system consists of The energy change of a reaction the kinetic energy of all the particles (related to is measured at constant temperature) plus the potential energy of volume (in a bomb interaction between the particles and within the calorimeter). particles (eg bonding). We can only measure the change in energy of the system (units = J or Nm). More conveniently reactions are performed at constant Energy pressure which measures enthalpy change, ∆H. initial state final state ∆H ~ ∆E for most reactions we study. final state initial state ∆H < 0 exothermic reaction Energy "lost" to surroundings Energy "gained" from surroundings ∆H > 0 endothermic reaction < 0 > 0 2 o Enthalpy of formation, fH Hess’s Law o Hess's Law: The heat change in any reaction is the The standard enthalpy of formation, fH , is the change in enthalpy when one mole of a substance is formed from same whether the reaction takes place in one step or its elements under a standard pressure of 1 atm. several steps, i.e. the overall energy change of a reaction is independent of the route taken. The heat of formation of any element in its standard state is defined as zero. o The standard enthalpy of reaction, H , is the sum of the enthalpy of the products minus the sum of the enthalpy of the reactants. Start End o o o H = prod nfH - react nfH 3 4 Example Application – energy foods! Calculate Ho for CH (g) + 2O (g) CO (g) + 2H O(l) Do you get more energy from the metabolism of 1.0 g of sugar or -

Thermodynamic Potential of Free Energy for Thermo-Elastic-Plastic Body
Continuum Mech. Thermodyn. (2018) 30:221–232 https://doi.org/10.1007/s00161-017-0597-3 ORIGINAL ARTICLE Z. Sloderbach´ · J. Paja˛k Thermodynamic potential of free energy for thermo-elastic-plastic body Received: 22 January 2017 / Accepted: 11 September 2017 / Published online: 22 September 2017 © The Author(s) 2017. This article is an open access publication Abstract The procedure of derivation of thermodynamic potential of free energy (Helmholtz free energy) for a thermo-elastic-plastic body is presented. This procedure concerns a special thermodynamic model of a thermo-elastic-plastic body with isotropic hardening characteristics. The classical thermodynamics of irre- versible processes for material characterized by macroscopic internal parameters is used in the derivation. Thermodynamic potential of free energy may be used for practical determination of the level of stored energy accumulated in material during plastic processing applied, e.g., for industry components and other machinery parts received by plastic deformation processing. In this paper the stored energy for the simple stretching of austenitic steel will be presented. Keywords Free energy · Thermo-elastic-plastic body · Enthalpy · Exergy · Stored energy of plastic deformations · Mechanical energy dissipation · Thermodynamic reference state 1 Introduction The derivation of thermodynamic potential of free energy for a model of the thermo-elastic-plastic body with the isotropic hardening is presented. The additive form of the thermodynamic potential is assumed, see, e.g., [1–4]. Such assumption causes that the influence of plastic strains on thermo-elastic properties of the bodies is not taken into account, meaning the lack of “elastic–plastic” coupling effects, see Sloderbach´ [4–10].