Mirna Detection Using a Rolling Circle Amplification and RNA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme
University of Central Florida STARS HIM 1990-2015 2015 Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme Amanda J. Cox University of Central Florida Part of the Biochemistry Commons Find similar works at: https://stars.library.ucf.edu/honorstheses1990-2015 University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by STARS. It has been accepted for inclusion in HIM 1990-2015 by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Cox, Amanda J., "Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme" (2015). HIM 1990-2015. 1861. https://stars.library.ucf.edu/honorstheses1990-2015/1861 USING ANTENNA TILE-ASSISTED SUBSTRATE DELIVERY TO IMPROVE THE DETECTION LIMITS OF DEOXYRIBOZYME BIOSENSORS by AMANDA J. COX A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Chemistry, Biochemistry Track in the College of Sciences and in the Burnett Honors College at the University of Central Florida Orlando, Florida Fall Term, 2015 Thesis Chair: Dr. Dmitry Kolpashchikov, PhD ABSTRACT One common limitation of enzymatic reactions is the diffusion of a substrate to the enzyme active site and/or the release of the reaction products. These reactions are known as diffusion – controlled. Overcoming this limitation may enable faster catalytic rates, which in the case of catalytic biosensors can potentially lower limits of detection of specific analyte. Here we created an artificial system to enable deoxyribozyme (Dz) 10-23 based biosensor to overcome its diffusion limit. -
Eukaryotic Non-Coding Rnas: New Targets for Diagnostics and Therapeutics?
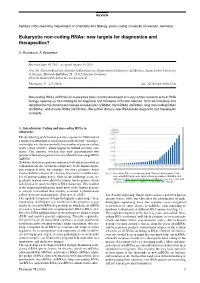
REVIEW Institute of Biochemistry, Department of Chemistry and Biology, Justus Liebig University of Giessen, Germany Eukaryotic non-coding RNAs: new targets for diagnostics and therapeutics? O. Rossbach, A. Bindereif Received June 30, 2015, accepted August 19, 2015 Prof. Dr. Albrecht Bindereif, Institute of Biochemistry, Department of Chemistry and Biology, Justus Liebig University of Giessen, Heinrich-Buff-Ring 58, 35392 Giessen, Germany [email protected] Pharmazie 71: 3–7 (2016) doi: 10.1691/ph.2016.5736 Non-coding RNAs (ncRNAs) in eukaryotes have recently developed to a very active research area in RNA biology, opening up new strategies for diagnosis and therapies of human disease. Here we introduce and describe the most important classes of eukaryotic ncRNAs: microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). We further discuss new RNA-based diagnostic and therapeutic concepts. 1. Introduction: Coding and non-coding RNAs in eukaryotes The deciphering of the human genome sequence in 2000 marked a unique breakthrough in modern molecular biology. An impor- tant insight was the unexpectedly low number of protein-coding genes (about 20,000), which lagged far behind previous esti- mates. This number revealed that only approximately two percent of the human genome is transcribed into messenger RNA (mRNA). However, the human genome sequence itself did not provide an explanation for the enormous complexity of the human organ- ism compared with, for example, the more primitive worm Caenorhabditis elegans (C. elegans) that carries a similar num- Fig. 1: Non-coding RNAs as an emerging field. The rapid development of the ber of protein-coding genes. -

Split Deoxyribozyme Probe for Efficient Detection of Highly Structured RNA Targets
University of Central Florida STARS Honors Undergraduate Theses UCF Theses and Dissertations 2018 Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets Sheila Raquel Solarez University of Central Florida Part of the Biochemistry Commons, and the Biology Commons Find similar works at: https://stars.library.ucf.edu/honorstheses University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by the UCF Theses and Dissertations at STARS. It has been accepted for inclusion in Honors Undergraduate Theses by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Solarez, Sheila Raquel, "Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets" (2018). Honors Undergraduate Theses. 311. https://stars.library.ucf.edu/honorstheses/311 SPLIT DEOXYRIBOZYME PROBE FOR EFFICIENT DETECTION OF HIGHLY STRUCTURED RNA TARGETS By SHEILA SOLAREZ A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Biological Sciences in the College of Sciences and the Burnett Honors College at the University of Central Florida Orlando, Florida Spring Term, 2018 Thesis Chair: Yulia Gerasimova, PhD ABSTRACT Transfer RNAs (tRNAs) are known for their role as adaptors during translation of the genetic information and as regulators for gene expression; uncharged tRNAs regulate global gene expression in response to changes in amino acid pools in the cell. Aminoacylated tRNAs play a role in non-ribosomal peptide bond formation, post-translational protein labeling, modification of phospholipids in the cell membrane, and antibiotic biosynthesis. [1] tRNAs have a highly stable structure that can present a challenge for their detection using conventional techniques. -

Synthetic Biology Applying Engineering to Biology
Synthetic Biology Applying Engineering to Biology Report of a NEST High-Level Expert Group EUR 21796 PROJECT REPORT Interested in European research? RTD info is our quarterly magazine keeping you in touch with main developments (results, programmes, events, etc). It is available in English, French and German. A free sample copy or free subscription can be obtained from: European Commission Directorate-General for Research Information and Communication Unit B-1049 Brussels Fax : (32-2) 29-58220 E-mail: [email protected] Internet: http://europa.eu.int/comm/research/rtdinfo/index_en.html EUROPEAN COMMISSION Directorate-General for Research Directorate B — Structuring the European Research Area Unit B1 — Anticipation of Scientific and Technological Needs (NEST activity); Basic Research E-mail: [email protected] Contact: Christian Krassnig European Commission Office SDME 01/37 B-1049 Brussels Tel. (32-2) 29-86445 Fax (32-2) 29-93173 E-mail: [email protected] For further information on the NEST activity please refer to the following website: http://www.cordis.lu/nest/home.html EUROPEAN COMMISSION Synthetic Biology Applying Engineering to Biology Report of a NEST High-Level Expert Group NEST - New and Energing Science and Technology - is a research activity under the European Community’s 6th Framework Programme Directorate-General for Research Structuring the European Research Area 2005 Anticipating Scientific and Technological Needs; Basic Research EUR 21796 Europe Direct is a service to help you find answers to your questions about the European Union Freephone number: 00 800 6 7 8 9 10 11 LEGAL NOTICE: Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub
US 20040O86860A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub. Date: May 6, 2004 (54) METHODS OF PRODUCING RNAS OF Publication Classification DEFINED LENGTH AND SEQUENCE (51) Int. Cl." .............................. C12Q 1/68; C12P 19/34 (76) Inventor: Muhammad Sohail, Marston (GB) (52) U.S. Cl. ............................................... 435/6; 435/91.2 Correspondence Address: MINTZ, LEVIN, COHN, FERRIS, GLOWSKY (57) ABSTRACT AND POPEO, PC. ONE FINANCIAL CENTER Methods of making RNA duplexes and single-stranded BOSTON, MA 02111 (US) RNAS of a desired length and Sequence based on cleavage of RNA molecules at a defined position, most preferably (21) Appl. No.: 10/264,748 with the use of deoxyribozymes. Novel deoxyribozymes capable of cleaving RNAS including a leader Sequence at a (22) Filed: Oct. 4, 2002 Site 3' to the leader Sequence are also described. Patent Application Publication May 6, 2004 Sheet 1 of 2 US 2004/0086860 A1 DNA Oligonucleotides T7 Promoter -TN-- OR 2N-2-N-to y Transcription Products GGGCGAAT-N-UU GGGCGAAT-N-UU w N Deoxyribozyme Cleavage - Q GGGCGAAT -------' Racction GGGCGAAT N-- UU N-UU ssRNA products N-UU Anneal ssRNA UU S-2N- UU siRNA product FIGURE 1: Flowchart summarising the procedure for siRNA synthesis. Patent Application Publication May 6, 2004 Sheet 2 of 2 US 2004/0086860 A1 Full-length transcript 3'-digestion product 5'-digestion product (5'GGGCGAATA) A: Production of single-stranded RNA templates by in vitro transcription and digestion With a deoxyribozyme V 2- 2 V 22inv 22 * 2 &3 S/AS - 88.8x, *...* or as IGFR -- is as 4. -

A Pirna-Like Small RNA Interacts with and Modulates P-ERM Proteins in Human Somatic Cells
ARTICLE Received 18 Mar 2015 | Accepted 27 Apr 2015 | Published 22 June 2015 DOI: 10.1038/ncomms8316 OPEN A piRNA-like small RNA interacts with and modulates p-ERM proteins in human somatic cells Yuping Mei1, Yuyan Wang1,2, Priti Kumari3, Amol Carl Shetty3, David Clark1, Tyler Gable1, Alexander D. MacKerell4,5, Mark Z. Ma1, David J. Weber5,6, Austin J. Yang5, Martin J. Edelman5 & Li Mao1,5 PIWI-interacting RNAs (piRNAs) are thought to silence transposon and gene expression during development. However, the roles of piRNAs in somatic tissues are largely unknown. Here we report the identification of 555 piRNAs in human lung bronchial epithelial (HBE) and non-small cell lung cancer (NSCLC) cell lines, including 295 that do not exist in databases termed as piRNA-like sncRNAs or piRNA-Ls. Distinctive piRNA/piRNA-L expression patterns are observed between HBE and NSCLC cells. piRNA-like-163 (piR-L-163), the top down- regulated piRNA-L in NSCLC cells, binds directly to phosphorylated ERM proteins (p-ERM), which is dependent on the central part of UUNNUUUNNUU motif in piR-L-163 and the RRRKPDT element in ERM. The piR-L-163/p-ERM interaction is critical for p-ERM’s binding capability to filamentous actin (F-actin) and ERM-binding phosphoprotein 50 (EBP50). Thus, piRNA/piRNA-L may play a regulatory role through direct interaction with proteins in physiological and pathophysiological conditions. 1 Department of Oncology and Diagnostic Sciences, University of Maryland School of Dentistry, 650W Baltimore Street, Baltimore, Maryland 21201, USA. 2 Department of Thoracic Medical Oncology, Peking University Cancer Hospital and Institute, Beijing 100142, China. -

Hammerhead Ribozymes Against Virus and Viroid Rnas
Hammerhead Ribozymes Against Virus and Viroid RNAs Alberto Carbonell, Ricardo Flores, and Selma Gago Contents 1 A Historical Overview: Hammerhead Ribozymes in Their Natural Context ................................................................... 412 2 Manipulating Cis-Acting Hammerheads to Act in Trans ................................. 414 3 A Critical Issue: Colocalization of Ribozyme and Substrate . .. .. ... .. .. .. .. .. ... .. .. .. .. 416 4 An Unanticipated Participant: Interactions Between Peripheral Loops of Natural Hammerheads Greatly Increase Their Self-Cleavage Activity ........................... 417 5 A New Generation of Trans-Acting Hammerheads Operating In Vitro and In Vivo at Physiological Concentrations of Magnesium . ...... 419 6 Trans-Cleavage In Vitro of Short RNA Substrates by Discontinuous and Extended Hammerheads ........................................... 420 7 Trans-Cleavage In Vitro of a Highly Structured RNA by Discontinuous and Extended Hammerheads ........................................... 421 8 Trans-Cleavage In Vivo of a Viroid RNA by an Extended PLMVd-Derived Hammerhead ........................................... 422 9 Concluding Remarks and Outlooks ........................................................ 424 References ....................................................................................... 425 Abstract The hammerhead ribozyme, a small catalytic motif that promotes self- cleavage of the RNAs in which it is found naturally embedded, can be manipulated to recognize and cleave specifically -

Questions with Answers- Nucleotides & Nucleic Acids A. the Components
Questions with Answers- Nucleotides & Nucleic Acids A. The components and structures of common nucleotides are compared. (Questions 1-5) 1._____ Which structural feature is shared by both uracil and thymine? a) Both contain two keto groups. b) Both contain one methyl group. c) Both contain a five-membered ring. d) Both contain three nitrogen atoms. 2._____ Which component is found in both adenosine and deoxycytidine? a) Both contain a pyranose. b) Both contain a 1,1’-N-glycosidic bond. c) Both contain a pyrimidine. d) Both contain a 3’-OH group. 3._____ Which property is shared by both GDP and AMP? a) Both contain the same charge at neutral pH. b) Both contain the same number of phosphate groups. c) Both contain the same purine. d) Both contain the same furanose. 4._____ Which characteristic is shared by purines and pyrimidines? a) Both contain two heterocyclic rings with aromatic character. b) Both can form multiple non-covalent hydrogen bonds. c) Both exist in planar configurations with a hemiacetal linkage. d) Both exist as neutral zwitterions under cellular conditions. 5._____ Which property is found in nucleosides and nucleotides? a) Both contain a nitrogenous base, a pentose, and at least one phosphate group. b) Both contain a covalent phosphodister bond that is broken in strong acid. c) Both contain an anomeric carbon atom that is part of a β-N-glycosidic bond. d) Both contain an aldose with hydroxyl groups that can tautomerize. ___________________________________________________________________________ B. The structures of nucleotides and their components are studied. (Questions 6-10) 6._____ Which characteristic is shared by both adenine and cytosine? a) Both contain one methyl group. -

Neo-Cloo2 B2 O 1 B 1 (3) REVERSE TRANSCRIPTION RNA PROCESSING
|||||||||||||| O US005436141A United States Patent (19) 11 Patent Number: 5,436,141 Miyata et al. (45) Date of Patent: Jul. 25, 1995 54 METHOD FOR SYNTHESIZING STABLE 56) References Cited SINGLE-STRANDED CONAN EUKARYOTES BY MEANS OF A U.S. PATENT DOCUMENTS BACTERAL RETRON AND PRODUCTS 5,079,151 1/1992 Lampson et al. ................ 435/91.51 75 Inventors: Shohei Miyata, Misato, Japan; FOREIGN PATENT DOCUMENTS Atsushi Ohshima, Highland Park, 0132309 1/1985 European Pat. Off. ... C12N 15/00 N.J.; Sumiko Inouye; Masayori Inouye, both of Bridgewater, N.J. OTHER PUBLICATIONS Y o Dhundale et al., J. Bact, vol. 170, 1988, pp. 5620-5624. 73) Assignee: University of Medicine and Dentistry Lampson et al., Science, vol. 243, 1989, pp. 1033-1038. of New Jersey, Newark, N.J. Sambrook et al., Molecular Cloning: A Laboratory Man ual, Cold Spring Harbor Laboratory Press, 1989, pp. 21 Appl. No.: 753,110 16.15-16.16. 22 Filed: Aug. 30, 1991 Primary Examiner-Richard A. Schwartz Assistant Examiner-James Ketter Related U.S. Application Data Attorney, Agent, or Firm-Weiser & Associates (63) Continuation-in-part of Ser. No. 315,427, Feb. 24, 1989, 57 ABSTRACT Pat. No. 5,979,151, and a continuation in part of Ser. A method for producing in vivo stable single-stranded No. 315,316, Feb. 24, 1989, Pat. No. 5,320,958, and a DNAs in eucaryotic cells. The DNAs are multicopy Eup ii.g 1. single-stranded DNA (msDNA) structures constituted 517,946, May 2, 1990, and a continuation-in-part of Ser. by a RNA and a DNA portion. -

De Novo Nucleic Acids: a Review of Synthetic Alternatives to DNA and RNA That Could Act As † Bio-Information Storage Molecules
life Review De Novo Nucleic Acids: A Review of Synthetic Alternatives to DNA and RNA That Could Act as y Bio-Information Storage Molecules Kevin G Devine 1 and Sohan Jheeta 2,* 1 School of Human Sciences, London Metropolitan University, 166-220 Holloway Rd, London N7 8BD, UK; [email protected] 2 Network of Researchers on the Chemical Evolution of Life (NoR CEL), Leeds LS7 3RB, UK * Correspondence: [email protected] This paper is dedicated to Professor Colin B Reese, Daniell Professor of Chemistry, Kings College London, y on the occasion of his 90th Birthday. Received: 17 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Abstract: Modern terran life uses several essential biopolymers like nucleic acids, proteins and polysaccharides. The nucleic acids, DNA and RNA are arguably life’s most important, acting as the stores and translators of genetic information contained in their base sequences, which ultimately manifest themselves in the amino acid sequences of proteins. But just what is it about their structures; an aromatic heterocyclic base appended to a (five-atom ring) sugar-phosphate backbone that enables them to carry out these functions with such high fidelity? In the past three decades, leading chemists have created in their laboratories synthetic analogues of nucleic acids which differ from their natural counterparts in three key areas as follows: (a) replacement of the phosphate moiety with an uncharged analogue, (b) replacement of the pentose sugars ribose and deoxyribose with alternative acyclic, pentose and hexose derivatives and, finally, (c) replacement of the two heterocyclic base pairs adenine/thymine and guanine/cytosine with non-standard analogues that obey the Watson–Crick pairing rules. -

Manipulation of Gene Expression in Zebrafish Using Caged Circular Morpholino Oligomers Yuan Wang, Li Wu, Peng Wang, Cong Lv, Zhenjun Yang and Xinjing Tang*
Published online 22 September 2012 Nucleic Acids Research, 2012, Vol. 40, No. 21 11155–11162 doi:10.1093/nar/gks840 Manipulation of gene expression in zebrafish using caged circular morpholino oligomers Yuan Wang, Li Wu, Peng Wang, Cong Lv, Zhenjun Yang and Xinjing Tang* State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, No. 38, Xueyuan Road, Beijing 100191, China Received May 18, 2012; Revised August 12, 2012; Accepted August 14, 2012 ABSTRACT strategy with the attachment of multiple caging moieties to the nucleobases or the backbone of phosphate groups. Morpholino oligomers (MOs) have been widely used Ando et al. caged a green fluorescent protein (GFP) to knock down specific genes in zebrafish, but their mRNA and a DNA plasmid through non-specific constitutive activities limit their experimental appli- labeling of the backbone phosphates approximately once cations for studying a gene with multiple functions every 35 bases with coumarin-based photolabile protecting or within a gene network. We report herein a new groups. These caged mRNA and DNA constructs enabled design and synthesis of caged circular MOs (caged the photoregulation of GFP expression in zebrafish and cMOs) with two ends linked by a photocleavable subsequent study of the role of lhx2 in zebrafish forebrain moiety. These caged cMOs were successfully used growth (49,52). More recently, Deiters et al. reported to photomodulate b-catenin-2 and no tail expres- morpholino oligomers (MOs) with multiple photocaged sion in zebrafish embryos. monomeric building blocks and demonstrated the utility of these caged MOs in light-activated control of gene function in zebrafish embryos (26). -

Design and Switch of Catalytic Activity with the Dnazyme–Rnazyme Combination
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 581 (2007) 1763–1768 Design and switch of catalytic activity with the DNAzyme–RNAzyme combination Yongjie Sheng, Zhen Zeng, Wei Peng, Dazhi Jiang, Shuang Li, Yanhong Sun, Jin Zhang* Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, Jilin University, Changchun 130023, PR China Received 12 January 2007; revised 9 March 2007; accepted 16 March 2007 Available online 2 April 2007 Edited by Judit Ova´di backbone DNA with a regulating sequence [12]. Compared to Abstract Design and switch of catalytic activity in enzymology remains a subject of intense investigation. Here, we employ a the linear hammerhead ribozyme, the stability of the circular DNAzyme–RNAzyme combination strategy for construction of RNA–DNA enzyme was substantially enhanced. Furthering a 10–23 deoxyribozyme-hammerhead ribozyme combination that this approach, we substituted the hammerhead ribozyme with targets different sites of the b-lactamase mRNA. The 10–23 the more efficient and more stable 10–23 deoxyribozyme, while deoxyribozyme-hammerhead ribozyme combination gene was the backbone DNA was replaced by the single-stranded repli- cloned into phagemid vector pBlue-scriptIIKS (+). In vitro the cation-competent vector M13mp18 (7.25 kb), thus successfully single-strand recombinant phagemid vector containing the com- constructing a novel replicating circular deoxyribozyme, which bination sequence exhibited 10–23 deoxyribozyme activity, and displayed 10–23 deoxyribozyme activities both in vitro and in the linear transcript displayed hammerhead ribozyme activity. bacteria [13,14]. In bacteria, the 10–23 deoxyribozyme-hammerhead ribozyme Here, we report the combination of 10–23 deoxyribozyme combination inhibited the b-lactamase expression and repressed the growth of drug-resistant bacteria.