Split Deoxyribozyme Probe for Efficient Detection of Highly Structured RNA Targets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme
University of Central Florida STARS HIM 1990-2015 2015 Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme Amanda J. Cox University of Central Florida Part of the Biochemistry Commons Find similar works at: https://stars.library.ucf.edu/honorstheses1990-2015 University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by STARS. It has been accepted for inclusion in HIM 1990-2015 by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Cox, Amanda J., "Using Antenna Tile-Assisted Substrate Delivery to Improve Detection Limits of Deoxyribozyme" (2015). HIM 1990-2015. 1861. https://stars.library.ucf.edu/honorstheses1990-2015/1861 USING ANTENNA TILE-ASSISTED SUBSTRATE DELIVERY TO IMPROVE THE DETECTION LIMITS OF DEOXYRIBOZYME BIOSENSORS by AMANDA J. COX A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Chemistry, Biochemistry Track in the College of Sciences and in the Burnett Honors College at the University of Central Florida Orlando, Florida Fall Term, 2015 Thesis Chair: Dr. Dmitry Kolpashchikov, PhD ABSTRACT One common limitation of enzymatic reactions is the diffusion of a substrate to the enzyme active site and/or the release of the reaction products. These reactions are known as diffusion – controlled. Overcoming this limitation may enable faster catalytic rates, which in the case of catalytic biosensors can potentially lower limits of detection of specific analyte. Here we created an artificial system to enable deoxyribozyme (Dz) 10-23 based biosensor to overcome its diffusion limit. -

Synthetic Biology Applying Engineering to Biology
Synthetic Biology Applying Engineering to Biology Report of a NEST High-Level Expert Group EUR 21796 PROJECT REPORT Interested in European research? RTD info is our quarterly magazine keeping you in touch with main developments (results, programmes, events, etc). It is available in English, French and German. A free sample copy or free subscription can be obtained from: European Commission Directorate-General for Research Information and Communication Unit B-1049 Brussels Fax : (32-2) 29-58220 E-mail: [email protected] Internet: http://europa.eu.int/comm/research/rtdinfo/index_en.html EUROPEAN COMMISSION Directorate-General for Research Directorate B — Structuring the European Research Area Unit B1 — Anticipation of Scientific and Technological Needs (NEST activity); Basic Research E-mail: [email protected] Contact: Christian Krassnig European Commission Office SDME 01/37 B-1049 Brussels Tel. (32-2) 29-86445 Fax (32-2) 29-93173 E-mail: [email protected] For further information on the NEST activity please refer to the following website: http://www.cordis.lu/nest/home.html EUROPEAN COMMISSION Synthetic Biology Applying Engineering to Biology Report of a NEST High-Level Expert Group NEST - New and Energing Science and Technology - is a research activity under the European Community’s 6th Framework Programme Directorate-General for Research Structuring the European Research Area 2005 Anticipating Scientific and Technological Needs; Basic Research EUR 21796 Europe Direct is a service to help you find answers to your questions about the European Union Freephone number: 00 800 6 7 8 9 10 11 LEGAL NOTICE: Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. -

Mrna Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection
International Journal of Molecular Sciences Review mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection 1, 1, 2 1,3, Shuqin Xu y, Kunpeng Yang y, Rose Li and Lu Zhang * 1 State Key Laboratory of Genetic Engineering, Institute of Genetics, School of Life Science, Fudan University, Shanghai 200438, China; [email protected] (S.X.); [email protected] (K.Y.) 2 M.B.B.S., School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; [email protected] 3 Shanghai Engineering Research Center of Industrial Microorganisms, Shanghai 200438, China * Correspondence: [email protected]; Tel.: +86-13524278762 These authors contributed equally to this work. y Received: 30 July 2020; Accepted: 30 August 2020; Published: 9 September 2020 Abstract: Messenger ribonucleic acid (mRNA)-based drugs, notably mRNA vaccines, have been widely proven as a promising treatment strategy in immune therapeutics. The extraordinary advantages associated with mRNA vaccines, including their high efficacy, a relatively low severity of side effects, and low attainment costs, have enabled them to become prevalent in pre-clinical and clinical trials against various infectious diseases and cancers. Recent technological advancements have alleviated some issues that hinder mRNA vaccine development, such as low efficiency that exist in both gene translation and in vivo deliveries. mRNA immunogenicity can also be greatly adjusted as a result of upgraded technologies. In this review, we have summarized details regarding the optimization of mRNA vaccines, and the underlying biological mechanisms of this form of vaccines. Applications of mRNA vaccines in some infectious diseases and cancers are introduced. It also includes our prospections for mRNA vaccine applications in diseases caused by bacterial pathogens, such as tuberculosis. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub
US 20040O86860A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub. Date: May 6, 2004 (54) METHODS OF PRODUCING RNAS OF Publication Classification DEFINED LENGTH AND SEQUENCE (51) Int. Cl." .............................. C12Q 1/68; C12P 19/34 (76) Inventor: Muhammad Sohail, Marston (GB) (52) U.S. Cl. ............................................... 435/6; 435/91.2 Correspondence Address: MINTZ, LEVIN, COHN, FERRIS, GLOWSKY (57) ABSTRACT AND POPEO, PC. ONE FINANCIAL CENTER Methods of making RNA duplexes and single-stranded BOSTON, MA 02111 (US) RNAS of a desired length and Sequence based on cleavage of RNA molecules at a defined position, most preferably (21) Appl. No.: 10/264,748 with the use of deoxyribozymes. Novel deoxyribozymes capable of cleaving RNAS including a leader Sequence at a (22) Filed: Oct. 4, 2002 Site 3' to the leader Sequence are also described. Patent Application Publication May 6, 2004 Sheet 1 of 2 US 2004/0086860 A1 DNA Oligonucleotides T7 Promoter -TN-- OR 2N-2-N-to y Transcription Products GGGCGAAT-N-UU GGGCGAAT-N-UU w N Deoxyribozyme Cleavage - Q GGGCGAAT -------' Racction GGGCGAAT N-- UU N-UU ssRNA products N-UU Anneal ssRNA UU S-2N- UU siRNA product FIGURE 1: Flowchart summarising the procedure for siRNA synthesis. Patent Application Publication May 6, 2004 Sheet 2 of 2 US 2004/0086860 A1 Full-length transcript 3'-digestion product 5'-digestion product (5'GGGCGAATA) A: Production of single-stranded RNA templates by in vitro transcription and digestion With a deoxyribozyme V 2- 2 V 22inv 22 * 2 &3 S/AS - 88.8x, *...* or as IGFR -- is as 4. -

Expanding the Genetic Code Lei Wang and Peter G
Reviews P. G. Schultz and L. Wang Protein Science Expanding the Genetic Code Lei Wang and Peter G. Schultz* Keywords: amino acids · genetic code · protein chemistry Angewandte Chemie 34 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/anie.200460627 Angew. Chem. Int. Ed. 2005, 44,34–66 Angewandte Protein Science Chemie Although chemists can synthesize virtually any small organic molecule, our From the Contents ability to rationally manipulate the structures of proteins is quite limited, despite their involvement in virtually every life process. For most proteins, 1. Introduction 35 modifications are largely restricted to substitutions among the common 20 2. Chemical Approaches 35 amino acids. Herein we describe recent advances that make it possible to add new building blocks to the genetic codes of both prokaryotic and 3. In Vitro Biosynthetic eukaryotic organisms. Over 30 novel amino acids have been genetically Approaches to Protein encoded in response to unique triplet and quadruplet codons including Mutagenesis 39 fluorescent, photoreactive, and redox-active amino acids, glycosylated 4. In Vivo Protein amino acids, and amino acids with keto, azido, acetylenic, and heavy-atom- Mutagenesis 43 containing side chains. By removing the limitations imposed by the existing 20 amino acid code, it should be possible to generate proteins and perhaps 5. An Expanded Code 46 entire organisms with new or enhanced properties. 6. Outlook 61 1. Introduction The genetic codes of all known organisms specify the same functional roles to amino acid residues in proteins. Selectivity 20 amino acid building blocks. These building blocks contain a depends on the number and reactivity (dependent on both limited number of functional groups including carboxylic steric and electronic factors) of a particular amino acid side acids and amides, a thiol and thiol ether, alcohols, basic chain. -
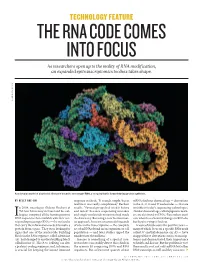
THE RNA CODE COMES INTO FOCUS As Researchers Open up to the Reality of RNA Modification, an Expanded Epitranscriptomics Toolbox Takes Shape
TECHNOLOGY FEATURE THE RNA CODE COMES INTO FOCUS As researchers open up to the reality of RNA modification, an expanded epitranscriptomics toolbox takes shape. LAGUNA DESIGN/SPL LAGUNA A molecular model of a bacterial ribosome bound to messenger RNA, a complex that is formed during protein synthesis. BY KELLY RAE CHI response in check. “It sounds simple, but in mRNAs harbour chemical tags — decorations real life it was really complicated,” Rechavi to the A, C, G and U nucleotides — that are n 2004, oncologist Gideon Rechavi at recalls. “Several groups had tried it before invisible to today’s sequencing technologies. Tel Aviv University in Israel and his col- and failed” because sequencing mistakes (Similar chemical tags, called epigenetic mark- leagues compared all the human genomic and single-nucleotide mutations had made ers, are also found on DNA.) Researchers aren’t IDNA sequences then available with their cor- the data noisy. But using a new bioinformat- sure what these chemical changes in RNA do, responding messenger RNAs — the molecules ics approach, his team uncovered thousands but they’re trying to find out. that carry the information needed to make a of sites in the transcriptome — the complete A wave of studies over the past five years — protein from a gene. They were looking for set of mRNAs found in an organism or cell many of which focus on a specific RNA mark signs that one of the nucleotide building population — and later studies upped the called N6-methyladenosine (m6A) — have blocks in the RNA sequence, called adenosine number into the millions1. -

Mrna, Rrna and Trna Types of RNA: Mrna, Rrna and Trna
Types of RNA: mRNA, rRNA and tRNA Types of RNA: mRNA, rRNA and tRNA By Susha Cheriyedath, M.Sc. Reviewed by Michael Greenwood, M.Sc. RNA or ribonucleic acid is a polymer of nucleotides that is made up of a ribose sugar, a phosphate, and bases such as adenine, guanine, cytosine, and uracil. It plays a crucial role in gene expression by acting as the intermediate between the genetic information encoded by DNA and proteins. Designua | Shutterstock RNA has a structure very similar to that of DNA. The key difference in RNA structure is that the ribose sugar in RNA possesses a hydroxyl (OH) group that is absent in DNA. Types of RNA In both prokaryotes and eukaryotes, there are three main types of RNA – messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). These 3 types of RNA are discussed below. P Saved from URL: https://www.news-medical.net/life-sciences/-Types-of-RNA-mRNA-rRNA-and-tRNA.aspx 1/5 Types of RNA: mRNA, rRNA and tRNA Messenger RNA (mRNA) mRNA accounts for just 5% of the total RNA in the cell. mRNA is the most heterogeneous of the 3 types of RNA in terms of both base sequence and size. It carries complimentary genetic code copied, from DNA during transcription, in the form of triplets of nucleotides called codons. Each codon specifies a particular amino acid, though one amino acid may be coded for by many different codons. Although there are 64 possible codons or triplet bases in the genetic code, only 20 of them represent amino acids. -

Transcription Study Guide This Study Guide Is a Written Version of the Material You Have Seen Presented in the Transcription Unit
Transcription Study Guide This study guide is a written version of the material you have seen presented in the transcription unit. The cell’s DNA contains the instructions for carrying out the work of the cell. These instructions are used by the cell’s protein-making machinery to create proteins. If the cell’s DNA were directly read by the protein-making machinery, however, it could be damaged and the process would be slow and cumbersome. The cell avoids this problem by copying genetic information from its DNA into an intermediate called messenger RNA (mRNA). It is this mRNA that is read by the cell’s protein-making machinery. This process is called transcription. Components In this section you will be introduced to the components involved in the process of RNA synthesis, called transcription. This process requires an enzyme that uses many nucleotide bases to copy the instructions present in DNA into an intermediate messenger RNA molecule. RNA What is RNA? · Like DNA, RNA is a polymer made up of nucleotides. · Unlike DNA, which is composed of two strands of nucleotides twisted together, RNA is single-stranded. It can also sometimes fold into complex three-dimensional structures. · RNA contains the same nucleotides as DNA, with the substitution of uraciluridine (U) for thymidine (T). · RNA is chemically different from DNA so that the cell can easily tell the two apart. · In this animation, you will see one type of RNA, messenger RNA, being put together. · There are three types of RNA: mRNA, which you will read more about; tRNA, which is used in the translation process, and rRNA, which acts as a structural element in the ribosome (a translation component). -

Isolation of RNA with Properties of Messenger RNA from Cerebral Polyribosomes* Claire E
Proceedings of the National Academy of Sciences Vol. 67, No. 2, pp. 644-651, October 1970 Isolation of RNA with Properties of Messenger RNA from Cerebral Polyribosomes* Claire E. Zomzelyt, Sidney Roberts, and Susan Peache DEPARTMENT OF BIOLOGICAL CHEMISTRY, SCHOOL OF MEDICINE, AND THE BRAIN RESEARCH INSTI- TUTE, UNIVERSITY OF CALIFORNIA CENTER FOR THE HEALTH SCIENCES, LOS ANGELES, CALIFORNIA 90024 Communicated by H. W. Magoun, July 24, 1970 Abstract. RNA which dissociated from purified cerebral polyribosomes of adult rats in the presence of EDTA was isolated by fractionation in a dis- continuous sucrose gradient. The yield was 2% of the total polyribosomal RNA. The base composition resembled the complementary values for rat DNA and was very different from base compositions of ribosomal RNA and transfer RNA. This RNA fraction contained a large proportion of molecules which were rapidly labeled in vivo and hybridized to homologous DNA. The polyribosomal RNA preparation also exhibited high template activity in a cerebral cell-free system which had previously been stripped of the capacity to incorporate amino acids in the absence of added messenger RNA (mRNA). Sedimentation analysis revealed only two peaks, with coefficients of approximately 8 S and 16 S. The data indicate that RNA with the properties of mRNA can be selectively isolated from cerebral polyribosomes under mild conditions which avoid degradation. Numerous studies support the concept that alterations in RNA and protein synthesis are associated with information transfer and storage in the central nervous system. Activation of the synthesis of specific proteins is suggested by the reports that new RNA with altered base composition, presumably mRNA, is formed during the acquisition phase of learning. -

Why We Should Take a Second Look at RNA Technology
RNA THERAPEUTICS RNA Modulation: Gabriele Campi Why we should take a second look at RNA technology As a scientist first, but also as an investor, I have always hereditary ATTR amyloidosis with polyneuropathy, an believed in the potential of RNA molecules as therapeutics. autosomal dominant neurodegenerative disease. In 2018 While most current drugs aim to stop disease by modulating patisiran (Onpattro) was approved by the FDA. It is existing proteins, RNA molecules operate one step earlier. delivered to cells by lipid nanoparticles rather than GalNAc In the case of RNA interference (RNAi), they modulate the (N-Acetylgalactoseamine-siRNA conjugates). genes involved in different pathological processes. The RNA Since the end of 2018, investors have made a cautious molecules, small interfering RNA and microRNA, both return to the RNA therapy space with a targeted funding degrade messenger RNA (mRNA) and prevent it from being of existing RNA companies. In parallel, new companies are translated into proteins. entering the arena. An unexpected boost to the industry has I am the co-founder of the investment company AurorA come from the development and approval of the first mRNA Science, which recently led a Series B financing round for the vaccines to treat Covid-19 from Pfizer/BioNTech and Moderna. Dutch company InteRNA Technologies, which is developing I have always believed in the potency of RNA interference RNA therapeutics for the treatment of advanced solid and modulation techniques that allow a researcher to target tumours. This is an ambitious project, but with a potential specific messenger RNAs through the use of siRNAs, or for success. -

Pfizer-Biontech COVID-19 Vaccine)
Nucleoside-modified messenger RNA (modmRNA) of SARS-CoV-2 Suspension for injection (Pfizer-BioNTech COVID-19 Vaccine) U.S. Trade Names Pfizer-BioNTech COVID-19 The list of names may not include all products that are available on the market. What is this medicine? SARS COVID-19 VACCINE (SARZ koh-vid 19 vak SEEN) is a vaccine used to reduce the risk of getting COVID-19. This vaccine does not treat COVID-19. There is no FDA-approved vaccine to prevent COVID-19. The FDA has authorized the emergency use of this vaccine during the COVID-19 pandemic. This medicine may be used for other purposes; ask your health care provider or pharmacist if you have questions. What should I tell my health care provider before I take this medicine? They need to know if you have any of these conditions: any allergies bleeding disorder fever or infection immune system problems recent or upcoming vaccine including previous COVID-19 vaccine an unusual or allergic reaction to COVID-19 vaccine, other drugs, foods, dyes, or preservatives pregnant or trying to get pregnant breast-feeding How should I use this medicine? This vaccine is injected into a muscle. It is given by a health care professional. A copy of the Fact Sheet for Recipients and Caregivers will be given before each vaccination. Be sure to read this information carefully each time. This sheet may change frequently. Talk to your health care provider about the use of this drug in children. Special care may be needed. Overdosage: If you think you have taken too much of this medicine contact a poison control center or emergency room at once. -

Mirna Detection Using a Rolling Circle Amplification and RNA
biosensors Article MiRNA Detection Using a Rolling Circle Amplification and RNA-Cutting Allosteric Deoxyribozyme Dual Signal Amplification Strategy Chenxin Fang, Ping Ouyang, Yuxing Yang, Yang Qing, Jialun Han, Wenyan Shang, Yubing Chen and Jie Du * State Key Laboratory of Marine Resource Utilization in South China Sea, College of Materials Science and Engineering, Hainan University, Haikou 570228, China; [email protected] (C.F.); [email protected] (P.O.); [email protected] (Y.Y.); [email protected] (Y.Q.); [email protected] (J.H.); [email protected] (W.S.); [email protected] (Y.C.) * Correspondence: [email protected] Abstract: A microRNA (miRNA) detection platform composed of a rolling circle amplification (RCA) system and an allosteric deoxyribozyme system is proposed, which can detect miRNA-21 rapidly and efficiently. Padlock probe hybridization with the target miRNA is achieved through complementary base pairing and the padlock probe forms a closed circular template under the action of ligase; this circular template results in RCA. In the presence of DNA polymerase, RCA proceeds and a long chain with numerous repeating units is formed. In the presence of single-stranded DNA (H1 and H2), multi-component nucleic acid enzymes (MNAzymes) are formed that have the ability to cleave substrates. Finally, substrates containing fluorescent and quenching groups and magnesium ions are added to the system to activate the MNAzyme and the substrate cleavage reaction, thus achieving fluorescence intensity amplification. The RCA–MNAzyme system has dual signal amplification and presents a sensing platform that demonstrates broad prospects in the analysis and detection of Citation: Fang, C.; Ouyang, P.; Yang, nucleic acids.