Hammerhead Ribozymes Against Virus and Viroid Rnas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sequence-Selective Recognition of Double-Stranded RNA And
Downloaded from rnajournal.cshlp.org on October 8, 2021 - Published by Cold Spring Harbor Laboratory Press Hnedzko et al. Sequence-Selective Recognition of Double-Stranded RNA and Enhanced Cellular Uptake of Cationic Nucleobase and Backbone- Modified Peptide Nucleic Acids Dziyana Hnedzko1,*, Dennis W. McGee,2 Yannis A. Karamitas1 and Eriks Rozners1,* Departments of 1 Chemistry and 2 Biological Sciences, Binghamton University, The State University of New York, Binghamton, New York 13902, United States. * To whom correspondence should be addressed. Tel: +1-607-777-2441; Fax: +1-607-777- 4478; Email: [email protected] and [email protected] Running Tittle: RNA recognition and cellular uptake of PNA 1 Downloaded from rnajournal.cshlp.org on October 8, 2021 - Published by Cold Spring Harbor Laboratory Press Hnedzko et al. ABSTRACT Sequence-selective recognition of complex RNAs in live cells could find broad applications in biology, biomedical research and biotechnology. However, specific recognition of structured RNA is challenging and generally applicable and effective methods are lacking. Recently, we found that peptide nucleic acids (PNAs) were unusually well suited ligands for recognition of double-stranded RNAs. Herein, we report that 2-aminopyridine (M) modified PNAs and their conjugates with lysine and arginine tripeptides form strong (Ka = 9.4 to 17 × 107 M-1) and sequence-selective triple helices with RNA hairpins at physiological pH and salt concentration. The affinity of PNA-peptide conjugates for the matched RNA hairpins was unusually high compared to the much lower affinity for DNA hairpins of the same 7 -1 sequence (Ka = 0.05 to 0.11 × 10 M ). The binding of double-stranded RNA by M-modified 4 -1 -1 PNA-peptide conjugates was a relatively fast process (kon = 2.9 × 10 M s ) compared to 3 -1 -1 the notoriously slow triple helix formation by oligodeoxynucleotides (kon ~ 10 M s ). -

Biophysical and Biochemical Investigations of RNA Catalysis in the Hammerhead Ribozyme
UC Santa Cruz UC Santa Cruz Previously Published Works Title Biophysical and biochemical investigations of RNA catalysis in the hammerhead ribozyme. Permalink https://escholarship.org/uc/item/366835vs Journal Quarterly reviews of biophysics, 32(3) ISSN 0033-5835 Author Scott, WG Publication Date 1999-08-01 DOI 10.1017/s003358350000353x Peer reviewed eScholarship.org Powered by the California Digital Library University of California Quarterly Reviews of Biophysics 32, 3 (1999), pp. 241–284 Printed in the United Kingdom 241 # 1999 Cambridge University Press Biophysical and biochemical investigations of RNA catalysis in the hammerhead ribozyme William G. Scott The Center for the Molecular Biology of RNA and the Department of Chemistry and Biochemistry, Sinsheimer Laboratories, University of California at Santa Cruz, Santa Cruz, California 95064, USA 1. How do ribozymes work? 241 2. The hammerhead RNA as a prototype ribozyme 242 2.1 RNA enzymes 242 2.2 Satellite self-cleaving RNAs 242 2.3 Hammerhead RNAs and hammerhead ribozymes 244 3. The chemical mechanism of hammerhead RNA self-cleavage 246 3.1 Phosphodiester isomerization via an SN2(P) reaction 247 3.2 The canonical role of divalent metal ions in the hammerhead ribozyme reaction 251 3.3 The hammerhead ribozyme does not actually require metal ions for catalysis 254 3.4 Hammerhead RNA enzyme kinetics 257 4. Sequence requirements for hammerhead RNA self-cleavage 260 4.1 The conserved core, mutagenesis and functional group modifications 260 4.2 Ground-state vs. transition-state effects 261 -
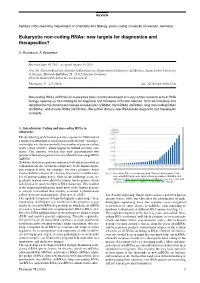
Eukaryotic Non-Coding Rnas: New Targets for Diagnostics and Therapeutics?
REVIEW Institute of Biochemistry, Department of Chemistry and Biology, Justus Liebig University of Giessen, Germany Eukaryotic non-coding RNAs: new targets for diagnostics and therapeutics? O. Rossbach, A. Bindereif Received June 30, 2015, accepted August 19, 2015 Prof. Dr. Albrecht Bindereif, Institute of Biochemistry, Department of Chemistry and Biology, Justus Liebig University of Giessen, Heinrich-Buff-Ring 58, 35392 Giessen, Germany [email protected] Pharmazie 71: 3–7 (2016) doi: 10.1691/ph.2016.5736 Non-coding RNAs (ncRNAs) in eukaryotes have recently developed to a very active research area in RNA biology, opening up new strategies for diagnosis and therapies of human disease. Here we introduce and describe the most important classes of eukaryotic ncRNAs: microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). We further discuss new RNA-based diagnostic and therapeutic concepts. 1. Introduction: Coding and non-coding RNAs in eukaryotes The deciphering of the human genome sequence in 2000 marked a unique breakthrough in modern molecular biology. An impor- tant insight was the unexpectedly low number of protein-coding genes (about 20,000), which lagged far behind previous esti- mates. This number revealed that only approximately two percent of the human genome is transcribed into messenger RNA (mRNA). However, the human genome sequence itself did not provide an explanation for the enormous complexity of the human organ- ism compared with, for example, the more primitive worm Caenorhabditis elegans (C. elegans) that carries a similar num- Fig. 1: Non-coding RNAs as an emerging field. The rapid development of the ber of protein-coding genes. -

The Variability of Hop Latent Viroid As Induced Upon Heat Treatment
Virology 287, 349–358 (2001) doi:10.1006/viro.2001.1044, available online at http://www.idealibrary.com on View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector The Variability of Hop Latent Viroid as Induced upon Heat Treatment Jaroslav Matousˇek,* Josef Patzak,† Lidmila Orctova´,* Jo¨rg Schubert,‡ Luka´sˇ Vrba,* Gerhard Steger,§ and Detlev Riesner§,1 *Department of Molecular Genetics, Institute of Plant Molecular Biology Czech Academy of Sciences, Branisˇovska´31, 37005 Cˇ eske´Bude˘jovice, Czech Republic; †Department of Virology, Institute of Hop Research and Breeding, Kadanˇska´2525, 438 46 Zˇatec, Czech Republic; ‡Federal Centre for Breeding Research, Institute for Resistance Research and Pathogen Diagnostics, Theodor-Roemer-Weg 4, 06449 Aschersleben, Germany; and §Institute of Physical Biology, Heinrich-Heine Universita¨t Du¨sseldorf, Universita¨tsstraße 1, D-40225 Du¨sseldorf, Germany Received March 28, 2001; returned to author for revision March 30, 2001; accepted June 11, 2001; published online August 2, 2001 We have previously shown that heat treatment of hop plants infected by hop latent viroid (HLVd) reduces viroid levels. Here we investigate whether such heat treatment leads to the accumulation of sequence variability in HLVd. We observed a negligible level of mutated variants in HLVd under standard cultivation conditions. In contrast, the heat treatment of hop led to HLVd degradation and, simultaneously, to a significant increase in sequence variations, as judged from temperature gradient–gel electrophoresis analysis and cDNA library screening by DNA heteroduplex analysis. Thirty-one cDNA clones (9.8%) were identified as deviating forms. -

How Might a Pre-Biotic Ribozyme Catalyze RNA Assembly in an RNA World?
Science Highlight – April 2007 How Might a Pre-biotic Ribozyme Catalyze RNA Assembly in an RNA World? Which came first, nucleic acids or proteins? This question is molecular biology's version of the "chicken-or-the-egg" riddle. Genes made of nucleic acids (DNA or RNA) contain the instructions for making proteins, but enzymes made of proteins are needed to replicate genes. For those who try to understand how life origi- nated, this once seemed an intractable paradox. The discovery 25 years ago that RNA can be enzymatic permits us to speculate that pre-biotic self-replicating molecules may have been RNAs (1,2). This is known as the "RNA World" hypothesis, and with the discovery of RNA catalysis, it is now possible to imagine a prebiotic The L1 Ligase ribozyme at the moment 'RNA World' (or even one populated by early life forms) of bond creation. in which self-replicating ribozymes (RNA-based enzymes that possess the catalytic ability to copy themselves) accomplished both tasks, thus avoiding the potential “chicken-or-the-egg” conundrum (3). But there's a catch. In order to copy RNA, fragments or monomers that have 5'-triphosphates must be ligated together. This is true for modern polymerases, and is also the most likely mechanism by which a ribozyme self-replicase in an RNA World might function. Yet no one has found a modern natural ribozyme that catalyze this The RNA nucleotide triphosphate ligation reaction required reaction (pictured right). for RNA polymerization and self-replication. RNA in vitro evolution and selection has however enabled several research groups to discover RNA sequences that can in fact cata- lyze the required chemical reaction (shown above) for 5'-triphos- phate RNA fragment ligation, and one group has even produced a primitive but functional RNA-based RNA polymerase ribozyme (4). -

Kinetic Characterization of Ribozymes Directed Against the Cisplatin
D2001 Nature Publishing Group 0929-1903/01/$17.00/+0 www.nature.com/cgt Kinetic characterization of ribozymes directed against the cisplatin resistance±associated ABC transporter cMOAT/MRP2/ABCC2 Verena Materna, Per Sonne Holm, Manfred Dietel, and Hermann Lage Institute of Pathology, Charite Campus Mitte, Humboldt University Berlin, Berlin D-10117, Germany. The enhanced expression of the human ABC transporter, cMOAT (MRP2/ABCC2), is associated with resistance of tumor cells against platinum-containing compounds, such as cisplatin. Therefore, cMOAT represents an interesting candidate factor for modulation of antineoplastic drug resistance. Two different hammerhead ribozymes, which exhibit high catalytic cleavage activities towards specific RNA sequences encoding cMOAT, were designed. Cleavage sites of these ribozymes are the GUC sites in codons 704 and 708 of the open readingframe in the cMOAT-specific mRNA molecule. Hammerhead ribozymes were in vitro synthesized using bacteriophage T7 RNA polymerase and oligonucleotide primers whereby one primer contains a T7 RNA polymerase promoter sequence. cMOAT-encodingsubstrate RNA molecules were created by a reverse transcription polymerase chain reaction usingRNA prepared from the cisplatin-resistant human ovarian carcinoma cell line A2780RCIS overexpressingthe cMOAT-encoding transcript. In a cell-free system, both anti-cMOAT ribozymes cleaved their substrate in a highly efficient manner at a physiologic pH and temperature. The cleavage reaction was dependent on time and ribozyme:substrate ratio for determining specific kinetic parameters. Cancer Gene Therapy (2001) 8, 176±184 Key words: cMOAT; MRP2; ABCC2; ribozyme; drug resistance; cisplatin. cquirement of resistance to antineoplastic agents, at of a broad range of endogenous and xenobiotic compounds Aconcentrations which were once effective for cancer across the apical canalicular membrane of the hepatocyte.4 chemotherapy, is a major obstacle in the clinical treatment Mutations in the cMOAT-encoding gene were shown to of human malignancies. -

A Tetracycline-Dependent Ribozyme Switch Allows Conditional Induction of Gene Expression in Caenorhabditis Elegans
ARTICLE https://doi.org/10.1038/s41467-019-08412-w OPEN A tetracycline-dependent ribozyme switch allows conditional induction of gene expression in Caenorhabditis elegans Lena A. Wurmthaler1,2, Monika Sack1,2, Karina Gense2,3, Jörg S. Hartig1,2 & Martin Gamerdinger2,3 The nematode Caenorhabditis elegans represents an important research model. Convenient methods for conditional induction of gene expression in this organism are not available. Here 1234567890():,; we describe tetracycline-dependent ribozymes as versatile RNA-based genetic switches in C. elegans. Ribozyme insertion into the 3’-UTR converts any gene of interest into a tetracycline- inducible gene allowing temporal and, by using tissue-selective promoters, spatial control of expression in all developmental stages of the worm. Using the ribozyme switches we established inducible C. elegans polyglutamine Huntington’s disease models exhibiting ligand- controlled polyQ-huntingtin expression, inclusion body formation, and toxicity. Our approach circumvents the complicated expression of regulatory proteins. Moreover, only little coding space is necessary and natural promoters can be utilized. With these advantages tetracycline-dependent ribozymes significantly expand the genetic toolbox for C. elegans. 1 Department of Chemistry, University of Konstanz, 78457 Konstanz, Germany. 2 Konstanz Research School Chemical Biology (KoRS-CB), University of Konstanz, 78457 Konstanz, Germany. 3 Department of Biology, University of Konstanz, 78457 Konstanz, Germany. Correspondence and requests for materials should be addressed to J.S.H. (email: [email protected]) or to M.G. (email: [email protected]) NATURE COMMUNICATIONS | (2019) 10:491 | https://doi.org/10.1038/s41467-019-08412-w | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-019-08412-w nducible regulatory systems are very powerful research tools to single A-to-G point mutation in the catalytic core of the HHR9 Iinvestigate the cellular function of individual genes. -

Nuclear and Mitochondrial DNA Sequences from Two Denisovan Individuals
Nuclear and mitochondrial DNA sequences from two Denisovan individuals Susanna Sawyera,1, Gabriel Renauda,1, Bence Violab,c,d, Jean-Jacques Hublinc, Marie-Theres Gansaugea, Michael V. Shunkovd,e, Anatoly P. Dereviankod,f, Kay Prüfera, Janet Kelsoa, and Svante Pääboa,2 aDepartment of Evolutionary Genetics, Max Planck Institute for Evolutionary Anthropology, D-04103 Leipzig, Germany; bDepartment of Anthropology, University of Toronto, Toronto, ON M5S 2S2, Canada; cDepartment of Human Evolution, Max Planck Institute for Evolutionary Anthropology, D-04103 Leipzig, Germany; dInstitute of Archaeology and Ethnography, Russian Academy of Sciences, Novosibirsk, RU-630090, Russia; eNovosibirsk National Research State University, Novosibirsk, RU-630090, Russia; and fAltai State University, Barnaul, RU-656049, Russia Contributed by Svante Pääbo, October 13, 2015 (sent for review April 16, 2015; reviewed by Hendrik N. Poinar, Fred H. Smith, and Chris B. Stringer) Denisovans, a sister group of Neandertals, have been described on DNA to the ancestors of present-day populations across Asia the basis of a nuclear genome sequence from a finger phalanx and Oceania suggests that in addition to the Altai Mountains, (Denisova 3) found in Denisova Cave in the Altai Mountains. The they may have lived in other parts of Asia. In addition to the only other Denisovan specimen described to date is a molar (Deni- finger phalanx, a molar (Denisova 4) was found in the cave in sova 4) found at the same site. This tooth carries a mtDNA se- 2000. Although less than 0.2% of the DNA in the tooth derives quence similar to that of Denisova 3. Here we present nuclear from a hominin source, the mtDNA was sequenced and differed DNA sequences from Denisova 4 and a morphological description, from the finger phalanx mtDNA at only two positions, suggesting as well as mitochondrial and nuclear DNA sequence data, from it too may be from a Denisovan (2, 3). -

Virus, Viroids and Mycoplasma
By: Dr. Bibha Kumari Dept. of Zoology Magadh Mahila College, Patna Email: [email protected] Virus •The viruses are non-cellular organisms. • They, in fact, have an inert crystalline structure outside the living cell. • Once they infect a cell, they take over the machinery of the host cell to replicate themselves, killing the host. •Pasteur. D.J. Ivanowsky (1892) gave the name virus. • It means venom or poisonous fluid. • According to his research, certain microbes caused the mosaic disease of tobacco. •These organisms were smaller than bacteria because they passed through bacteria-proof filters. • M.W. Beijerinek (1898) demonstrated that the extract of the infected plants of tobacco could cause infection in healthy plants. • He named the fluid as Contagium vivum fluidum (infectious living fluid). •W.M. Stanley (1935) discovered that viruses could be crystallized. These virus crystals are composed largely of proteins. •They are inert outside their specific host cell. Viruses are nothing but obligate parasites. Genetic Material of Viruses: •In addition to proteins, viruses also contain genetic material, that could be either RNA or DNA. • No virus contains both RNA and DNA. A virus is a nucleoprotein and the genetic material is infectious. •Speaking in strictly general terms, viruses infecting plants have single- stranded RNA. • On the other hand, viruses that infect animals have either single or double-stranded RNA or they might have double-stranded DNA •Bacterial viruses or bacteriophages usually have a double-stranded DNA structure. By bacteriophages, we mean viruses that infect the bacteria. • The protein coat, capsid made of small subunits (capsomeres) protects the nucleic acid. -

A Pirna-Like Small RNA Interacts with and Modulates P-ERM Proteins in Human Somatic Cells
ARTICLE Received 18 Mar 2015 | Accepted 27 Apr 2015 | Published 22 June 2015 DOI: 10.1038/ncomms8316 OPEN A piRNA-like small RNA interacts with and modulates p-ERM proteins in human somatic cells Yuping Mei1, Yuyan Wang1,2, Priti Kumari3, Amol Carl Shetty3, David Clark1, Tyler Gable1, Alexander D. MacKerell4,5, Mark Z. Ma1, David J. Weber5,6, Austin J. Yang5, Martin J. Edelman5 & Li Mao1,5 PIWI-interacting RNAs (piRNAs) are thought to silence transposon and gene expression during development. However, the roles of piRNAs in somatic tissues are largely unknown. Here we report the identification of 555 piRNAs in human lung bronchial epithelial (HBE) and non-small cell lung cancer (NSCLC) cell lines, including 295 that do not exist in databases termed as piRNA-like sncRNAs or piRNA-Ls. Distinctive piRNA/piRNA-L expression patterns are observed between HBE and NSCLC cells. piRNA-like-163 (piR-L-163), the top down- regulated piRNA-L in NSCLC cells, binds directly to phosphorylated ERM proteins (p-ERM), which is dependent on the central part of UUNNUUUNNUU motif in piR-L-163 and the RRRKPDT element in ERM. The piR-L-163/p-ERM interaction is critical for p-ERM’s binding capability to filamentous actin (F-actin) and ERM-binding phosphoprotein 50 (EBP50). Thus, piRNA/piRNA-L may play a regulatory role through direct interaction with proteins in physiological and pathophysiological conditions. 1 Department of Oncology and Diagnostic Sciences, University of Maryland School of Dentistry, 650W Baltimore Street, Baltimore, Maryland 21201, USA. 2 Department of Thoracic Medical Oncology, Peking University Cancer Hospital and Institute, Beijing 100142, China. -

Molecular Variability of Apple Hammerhead Viroid from Italian
Virus Research 270 (2019) 197644 Contents lists available at ScienceDirect Virus Research journal homepage: www.elsevier.com/locate/virusres Short communication Molecular variability of apple hammerhead viroid from Italian apple varieties supports the relevance in vivo of its branched conformation T stabilized by a kissing loop interaction Michela Chiumentia, Beatriz Navarroa, Pasquale Veneritob, Francesco Civitac, ⁎ ⁎ Angelantonio Minafraa, , Francesco Di Serioa, a Istituto per la Protezione Sostenibile delle Piante (CNR), Bari, Italy b Centro di Ricerca, Sperimentazione e Formazione in Agricoltura “Basile Caramia”, Locorotondo, Italy c SINAGRI – Università degli Studi di Bari “Aldo Moro”, Bari, Italy ARTICLE INFO ABSTRACT Keywords: In the absence of protein-coding ability, viroid RNAs rely on direct interactions with host factors for their Hammerhead infectivity. RNA structural elements are likely involved in these interactions. Therefore, preservation of a Non-Coding RNA structural element, despite the sequence variability existing between the variants of a viroid population, is Sequence variability considered a solid evidence of its relevant role in vivo. In this study, apple hammerhead viroid (AHVd) was first Secondary structure identified in the two apple cultivars ‘Mela Rosa Guadagno’ (MRG) and ‘Agostinella’ (AG), which are cultivated Co-Variation since long in Southern Italy, thus providing the first solid evidence of its presence in this country. Then, the Stem-Loop natural variability of AHVd viroid populations infecting MRG and AG was studied. The sequence variants from the two Italian isolates shared only 82.1–87.7% sequence identity with those reported previously from other geographic areas, thus providing the possibility of exploring the impact of this sequence divergence on the proposed secondary structure. -

Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120
atholog P y & nt a M Latifi et al., J Plant Pathol Microbiol 2016, 7:4 l i P c f r o o DOI: 10.4172/2157-7471.1000341 b l i Journal of a o l n o r g u y o J ISSN: 2157-7471 Plant Pathology & Microbiology Research Article Open Access Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120 Amel Latifi1*, Christophe Bernard1, Laura da Silva2, Yannick Andéol3, Amine Elleuch4, Véronique Risoul1, Jacques Vergne2 and Marie-Christine Maurel2 1Aix Marseille University, CNRS, UMR Chemistry Laboratory Bacterial 7283. 31 Chemin Joseph Aiguier 13009 Marseille Cedex 20, France 2Institute of Systematics, Evolution, Biodiversity ( ISyEB ), CNRS, MNHN , UPMC, EPHE, UPMC Sorbonne University, 57 rue Cuvier, PO Box 50, F-75005 Paris, France 3Team Enzymology of RNA, UR6, UPMC Sorbonne University, 75252 Paris Cedex 05 France 4Laboratory of Plant Biotechnology, Faculty of Sciences of Sfax, University of Sfax, BP 1171, 3000 Sfax, Tunisia Abstract Viroids are small infectious RNA molecules that replicate in plants via RNA-RNA replication processes. The molecular mechanism responsible for this replication has attracted great interest, and studies on this topic have yielded interesting biological findings on the processes in which RNA is involved. Viroids belonging to the Avsunviroidae family replicate in the chloroplasts of infected hosts. It has by now been established that chloroplasts and cyanobacteria share a common have ancestor. In view of this phylogenetic relationship, we investigated whether a member of the Avsunviroidae family could be replicated in a cyanobacterium. The results obtained here show that Avocado Sunblotch Viroid (ASBVd) RNA is able to replicate in the filamentous cyanobacterium Nostoc PCC 7120.