Rasopathies: Unraveling Mechanisms with Animal Models Granton A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
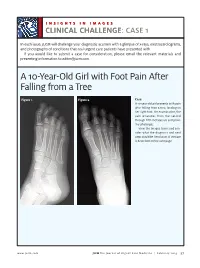
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Master List of References ACADVL 1
Last Revision: 6/2017 DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE PRECISION DIAGNOSTICS – INHERITED DISEASE Master List of References ACADVL 1. Mathur A, Sims HF, Gopalakrishnan D et al. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation 1999;99:1337-43. [PMID: 10077518] 2. Vianey-Saban C, Divry P, Brivet M et al. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: Clinical characteristics and diagnostic considerations in 30 patients. Clin Chim Acta 1998;269:43-62. [PMID: 9498103] AR 1. Giagulli VA, Carbone MD, De Pergola G, et al. Could androgen receptor gene CAG tract polymorphism affect spermatogenesis in men with idiopathic infertility? J Assist Reprod Genet 2014;31(6):689-97. [PMID: 24691874] 2. Gottlieb B, Beitel LK, Nadarajah A, et al. The androgen receptor gene mutations database (ARDB): 2012 update. Hum Mutat. 2012;33:887-94. [PMID: 22334387] Array CGH 1. Boone PM, Bacino CA, Shaw CA et al., Detection of clinically relevant exonic copy-number changes by array CGH. Hum Mutat 2010;31(12):1326-1342. [PMID: 20848651] CFTR 1. Collaco AM, Geibel P, Lee BS et al. Functional vacuolar ATPase (V-ATPase) proton pumps traffic to the enterocyte brush border membrane and require CFTR. Am J Physiol Cell Physiol 2013;305(9):C981-996. [PMID: 23986201] 2. Lu S, Yang X, Li X et al. Different cystic fibrosis transmembrane coductance regulator mutations in Chinese men with congenital bilateral absence of vas deferens and other acquired obtructive azoospermia. Urology 2013;82(4):824-828. [PMID: 23953609] 3. Sosnay PR, Siklosi KR, Van Goor F et al. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

International Symposium on New Developments in Neurofibromatoses and Rasopathies
INTERNATIONAL SYMPOSIUM ON NEW DEVELOPMENTS IN NEUROFIBROMATOSES AND RASOPATHIES Their Management, Diagnosis, Current and Future Therapeutic Targets 27th - 29th November 2017, Crowne Plaza, Kochi, Kerala, South India INTERNATIONAL SYMPOSIUM ON NEW DEVELOPMENTS IN NEUROFIBROMATOSES AND RASOPATHIES THE FIRST INTERNATIONAL SYMPOSIUM ON RASOPATHIES TO BE HELD IN ASIA RASopathies are a class of developmental disorders with overlapping clinical features as well as genetic mutations. RASopathies are associated with dysregulation of the RAS-MAPK (RAS/mitogen activated protein kinase) signalling pathway, an important pathway in humans as it is related to nine different genetic conditions and in many cancers. The meeting will bring together leading scientists, clinicians, researchers and trainee health care professionals in the field of genetics, cancer, neurology, paediatrics, cardiology, neurosurgery, craniofacial surgery, plastic surgery, dermatology, oncology and biomedical sciences from across the world. 200 25+ 20+ PLACES SPEAKERS TOPICS AIMS TO ENSURE THAT HEALTH PROFESSIONALS AND TO IDENTIFY THERAPEUTIC TARGETS FOR SCIENTISTS ARE KEPT ABREAST OF ANY NEW SEVERAL OF THESE RARE DISEASES DEVELOPMENTS IN CLINICAL PRACTICE TOPICS COVERED o RASopathies: Syndromes of Ras/ MAPK pathway o Cardio-Facio-Cutaneous Syndrome dysregulation o Capillary Malformation-Arteriovenous o Neurofibromatosis Type 1 Malformation Syndrome o Legius Syndrome o Natural history, improved diagnosis and genotype phenotype correlation for NF2 and o Noonan Syndrome Schwannomatosis -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

DGCR8 Microprocessor Defect Characterizes Familial Multinodular Goiter with Schwannomatosis
The Journal of Clinical Investigation CLINICAL MEDICINE DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis Barbara Rivera,1,2,3 Javad Nadaf,2,3 Somayyeh Fahiminiya,4 Maria Apellaniz-Ruiz,2,3,4,5 Avi Saskin,5,6 Anne-Sophie Chong,2,3 Sahil Sharma,7 Rabea Wagener,8 Timothée Revil,5,9 Vincenzo Condello,10 Zineb Harra,2,3 Nancy Hamel,4 Nelly Sabbaghian,2,3 Karl Muchantef,11,12 Christian Thomas,13 Leanne de Kock,2,3,5 Marie-Noëlle Hébert-Blouin,14 Angelia V. Bassenden,15 Hannah Rabenstein,8 Ozgur Mete,16,17 Ralf Paschke,18,19,20,21,22 Marc P. Pusztaszeri,23 Werner Paulus,13 Albert Berghuis,15 Jiannis Ragoussis,4,9 Yuri E. Nikiforov,10 Reiner Siebert,8 Steffen Albrecht,24 Robert Turcotte,25,26 Martin Hasselblatt,13 Marc R. Fabian,1,2,3,7,15 and William D. Foulkes1,2,3,4,5,6 1Gerald Bronfman Department of Oncology, McGill University, Montreal, Quebec, Canada. 2Lady Davis Institute for Medical Research and 3Segal Cancer Centre, Jewish General Hospital, Montreal, Quebec, Canada. 4Cancer Research Program, McGill University Health Centre, Montreal, Quebec, Canada. 5Department of Human Genetics, McGill University, Montreal, Quebec, Canada. 6Division of Medical Genetics, Department of Medicine, McGill University Health Centre and Jewish General Hospital, Montreal, Quebec, Canada. 7Department of Experimental Medicine, McGill University, Montreal, Quebec, Canada. 8Institute of Human Genetics, University of Ulm and University of Ulm Medical Center, Ulm, Germany. 9Génome Québec Innovation Centre, McGill University, Montreal, Quebec, Canada. 10Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA. 11Department of Diagnostic Radiology, McGill University, Montreal, Quebec, Canada. -

Costello Syndrome
orphananesthesia Anaesthesia recommendations for patients suffering from Costello syndrome Disease name: Costello syndrome ICD 10: Q87.8 Synonyms: Significant phenotypical overlap with CFC (cardiofaciocutaneous syndrome) and Noonan syndrome. Disease summary: Costello syndrome (CS) is a rare disorder (so-called RAS-opathy, see below), affecting up to 300 people worldwide. First described by Dr Jack Costello in 1977, the syndrome is characterised by failure to thrive (FTT), poor feeding, short stature, developmental delay, distinctive facial features, excessive loose skin, cardiac abnormalities, and an increased risk of tumour development. RAS is a family of genes coding for small GTPases and includes amongst others HRAS. The HRAS gene is a proto-oncogene, which forms part of the MAPK (mitogen activated protein kinase) signalling pathway. Up-regulation of this signalling pathway causes unopposed cell growth, causing tumour predisposition. The MAPK pathway is also the site of mutations causing both CFC and Noonan syndrome. CS can be caused by a number of mutations in the HRAS gene. Most mutations do occur de novo, but there is some evidence that a minority are inherited in an autosomal dominant manner. Patients with CS are born large for gestational age, and there is a strong association with polyhydramnios and preterm labour. Growth later slows due to feeding difficulties. Head circumference is affected to a lesser degree than height and weight, which gives rise to relative macrocephaly. Growth Hormone (GH) deficiency can cause neonatal hypoglycaemia, and contributes to growth retardation. The disease is characterised by distinctive facial features including downslanting palpebral fissures, epicanthic folds, ptosis, flattened nasal bridge (hypertelorism), low set ears, thick lips, macroglossia and short neck. -

Identification of Transcriptional Mechanisms Downstream of Nf1 Gene Defeciency in Malignant Peripheral Nerve Sheath Tumors Daochun Sun Wayne State University
Wayne State University DigitalCommons@WayneState Wayne State University Dissertations 1-1-2012 Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors Daochun Sun Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Recommended Citation Sun, Daochun, "Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors" (2012). Wayne State University Dissertations. Paper 558. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. IDENTIFICATION OF TRANSCRIPTIONAL MECHANISMS DOWNSTREAM OF NF1 GENE DEFECIENCY IN MALIGNANT PERIPHERAL NERVE SHEATH TUMORS by DAOCHUN SUN DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2012 MAJOR: MOLECULAR BIOLOGY AND GENETICS Approved by: _______________________________________ Advisor Date _______________________________________ _______________________________________ _______________________________________ © COPYRIGHT BY DAOCHUN SUN 2012 All Rights Reserved DEDICATION This work is dedicated to my parents and my wife Ze Zheng for their continuous support and understanding during the years of my education. I could not achieve my goal without them. ii ACKNOWLEDGMENTS I would like to express tremendous appreciation to my mentor, Dr. Michael Tainsky. His guidance and encouragement throughout this project made this dissertation come true. I would also like to thank my committee members, Dr. Raymond Mattingly and Dr. John Reiners Jr. for their sustained attention to this project during the monthly NF1 group meetings and committee meetings, Dr. -

Workshop Schedule
Workshop Schedule Friday, August 27th Registration: 4:00‐6:30 pm Dinner 6:30‐8:00 pm Reception 8:00‐10:00 Saturday, August 28th 7:00‐8:15 am Breakfast: 8:15am Welcome and Introductions 8:30‐10:15 am Session 1: Novel strategies to understand the causes/mechanisms of birth defects (Moderator: Angela Lin) 8:30 am Les Biesecker ‐ Using massively parallel sequencing technologies to provide improved molecular delineation of human malformation syndromes 9:15 am Bamshad ‐ Exome sequencing identifies a gene for Kabuki syndrome 9:30 am Boycott ‐ Next‐Generation sequencing strategies give insight into the mechanism of birth defects in a Canadian isolated population 9:45 am Bleyl ‐ Comparison of pooled allelic ratios (CoPAR) analysis: an efficient method for mapping genetic traits in extended pedigrees 10:00 am Allanson – Nablus mask‐like facial syndrome and blepharo‐naso‐facial syndrome are the same entity. Refinement of the critical region of chromosome 8q22.1 points to a potential candidate gene 10:15‐10:45 am BREAK 10:45‐12:00 pm Session 2: Novel strategies to understand the causes/mechanisms of birth defects (cont.) (Moderator: Mike Bamshad) 10:45 am Krantz ‐ Applying novel genomic tools towards understanding an old chromosomal diagnosis: Using genome‐wide expression and SNP genotyping to identify the true cause of Pallister‐Killian syndrome 11:00 am Paciorkowski ‐ Bioinformatic analysis of published and novel copy number variants suggests candidate genes and networks for infantile spasms 11:15 am Bernier ‐ Identification of a novel Fibulin -

S41467-021-24841-Y.Pdf
ARTICLE https://doi.org/10.1038/s41467-021-24841-y OPEN Integrative oncogene-dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer Athea Vichas1,10, Amanda K. Riley 1,2,10, Naomi T. Nkinsi1, Shriya Kamlapurkar1, Phoebe C. R. Parrish 1,3, April Lo1,3, Fujiko Duke4, Jennifer Chen4, Iris Fung4, Jacqueline Watson4, Matthew Rees 4, Austin M. Gabel 3,5,6,7, James D. Thomas 6,7, Robert K. Bradley 3,6,7, John K. Lee1, Emily M. Hatch 1,7, ✉ Marina K. Baine8, Natasha Rekhtman8, Marc Ladanyi8, Federica Piccioni4,9 & Alice H. Berger 1,3 1234567890():,; CRISPR-based cancer dependency maps are accelerating advances in cancer precision medicine, but adequate functional maps are limited to the most common oncogenes. To identify opportunities for therapeutic intervention in other rarer subsets of cancer, we investigate the oncogene-specific dependencies conferred by the lung cancer oncogene, RIT1. Here, genome-wide CRISPR screening in KRAS, EGFR, and RIT1-mutant isogenic lung cancer cells identifies shared and unique vulnerabilities of each oncogene. Combining this genetic data with small-molecule sensitivity profiling, we identify a unique vulnerability of RIT1- mutant cells to loss of spindle assembly checkpoint regulators. Oncogenic RIT1M90I weakens the spindle assembly checkpoint and perturbs mitotic timing, resulting in sensitivity to Aurora A inhibition. In addition, we observe synergy between mutant RIT1 and activation of YAP1 in multiple models and frequent nuclear overexpression of YAP1 in human primary RIT1-mutant lung tumors. These results provide a genome-wide atlas of oncogenic RIT1 functional inter- actions and identify components of the RAS pathway, spindle assembly checkpoint, and Hippo/YAP1 network as candidate therapeutic targets in RIT1-mutant lung cancer.