Workshop Schedule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
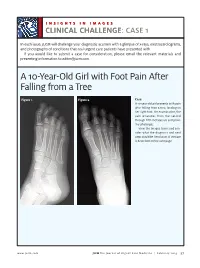
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

The Genetic Heterogeneity of Brachydactyly Type A1: Identifying the Molecular Pathways
The genetic heterogeneity of brachydactyly type A1: Identifying the molecular pathways Lemuel Jean Racacho Thesis submitted to the Faculty of Graduate Studies and Postdoctoral Studies in partial fulfillment of the requirements for the Doctorate in Philosophy degree in Biochemistry Specialization in Human and Molecular Genetics Department of Biochemistry, Microbiology and Immunology Faculty of Medicine University of Ottawa © Lemuel Jean Racacho, Ottawa, Canada, 2015 Abstract Brachydactyly type A1 (BDA1) is a rare autosomal dominant trait characterized by the shortening of the middle phalanges of digits 2-5 and of the proximal phalange of digit 1 in both hands and feet. Many of the brachymesophalangies including BDA1 have been associated with genetic perturbations along the BMP-SMAD signaling pathway. The goal of this thesis is to identify the molecular pathways that are associated with the BDA1 phenotype through the genetic assessment of BDA1-affected families. We identified four missense mutations that are clustered with other reported BDA1 mutations in the central region of the N-terminal signaling peptide of IHH. We also identified a missense mutation in GDF5 cosegregating with a semi-dominant form of BDA1. In two families we reported two novel BDA1-associated sequence variants in BMPR1B, the gene which codes for the receptor of GDF5. In 2002, we reported a BDA1 trait linked to chromosome 5p13.3 in a Canadian kindred (BDA1B; MIM %607004) but we did not discover a BDA1-causal variant in any of the protein coding genes within the 2.8 Mb critical region. To provide a higher sensitivity of detection, we performed a targeted enrichment of the BDA1B locus followed by high-throughput sequencing. -

The First Non-Invasive Prenatal Test That Screens for Single-Gene Disorders
The first non-invasive prenatal test that screens for single-gene disorders the evolution of NIPT A non-invasive prenatal test that screens multiple genes for mutations causing severe genetic disorders in the fetus analyses circulating cell- free fetal DNA (cfDNA) from a maternal blood sample. The test is performed after 10 weeks of pregnancy. works as a complementary screen to traditional and genome- wide NIPT . It screens for several life-altering genetic disorders that are not screened with current NIPT technology, allowing a complete picture of the risk of a pregnancy being affected by a genetic disorder. 2 facilitates early diagnosis of single-gene disorders. It involves 3 different levels of screening: This test screens for 5 common inherited recessive genetic disorders, such as Cystic Fibrosis, Beta-Thalassemia, Sickle INHERITED cell anaemia, Deafness autosomal recessive type 1A, Deafness autosomal recessive type 1B. Genes screened: CFTR, CX26 (GJB2), CX30 (GJB6), HBB This test screens for 44 severe genetic disorders due to de novo mutations (a gene mutation that is not inherited) in 25 genes DE NOVO Genes screened: ASXL1, BRAF, CBL, CHD7, COL1A1, COL1A2 , COL2A1, FGFR2, FGFR3, HDAC8, JAG1, KRAS, MAP2K1, MAP2K2, MECP2, NIPBL, NRAS, NSD1, PTPN11, RAF1, RIT1, SETBP1, SHOC2, SIX3, SOS1 This test screens for both inherited and de novo single-gene disorders and represents a combination of the tests INHERITED COMPLETE and DE NOVO providing a complete picture of the pregnancy risk. 3 allows detection of common inherited genetic disorders in INHERITED the fetus GENE GENETIC DISORDER CFTR Cystic Fibrosis CX26 (GJB2) Deafness autosomal recessive type 1A CX30 (GJB6) Deafness autosomal recessive type 1B HBB Beta-Thalassemia HBB Sickle cell anemia The inherited recessive disorders screened by INHERITED are the most common in the European population 4 identifies fetal conditions that could be missed by traditional DE NOVO prenatal screening. -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Metacarpophalangeal Pattern Profile Analysis in Sotos Syndrome
View metadata, citation and similar papers at core.ac.uk brought to you by CORE HHS Public Access provided by IUPUIScholarWorks Author manuscript Author ManuscriptAuthor Manuscript Author Am J Med Manuscript Author Genet. Author Manuscript Author manuscript; available in PMC 2017 September 12. Published in final edited form as: Am J Med Genet. 1985 April ; 20(4): 625–629. doi:10.1002/ajmg.1320200408. Metacarpophalangeal Pattern Profile Analysis in Sotos Syndrome Merlin G. Butler, Department of Biology, University of Notre Dame and North Central Regional Genetics Center, Memorial Hospital, South Bend, Indiana Department of Medical Genetics, Indiana University School of Medicine F. John Meaney, Department of Medical Genetics, Indiana University School of Medicine Genetic Diseases Section, Indiana State Board of Health, Indianapolis Smita Kittur, Pediatric Genetics Department, Children’s Medical and Surgical Center, Johns Hopkins Hospital, Baltimore Joseph H. Hersh, and Child Evaluation Center, Department of Pediatrics, University of Louisville School of Medicine Lusia Hornstein Cincinnati Center for Developmental Disorders, Children’s Hospital Medical Center Abstract The metacarpophalangeal pattern profile (MCPP) was analyzed on 16 Sotos syndrome patients. A mean Sotos syndrome profile was produced. Correlation studies confirm clinical homogeneity of Sotos syndrome individuals. Discriminant analysis of Sotos syndrome patients and normal individuals produces a function of two MCPP variables and age, which may provide a useful tool for diagnosis. Keywords Sotos syndrome; metacarpophalangeal pattern profile (MCPP); discriminant analysis; correlation studies INTRODUCTION Sotos syndrome, or cerebral gigantism, was first described by Sotos et al [1964]; at least 100 cases were reported subsequently. This syndrome is characterized by large size at birth, large hands and feet, advanced osseous maturation, macrocephaly with prominent forehead and Address reprint requests to Merlin G. -

Diagnostic Utility of Genome-Wide DNA Methylation Analysis in Mendelian Neurodevelopmental Disorders
International Journal of Molecular Sciences Review Diagnostic Utility of Genome-Wide DNA Methylation Analysis in Mendelian Neurodevelopmental Disorders Sadegheh Haghshenas 1,2 , Pratibha Bhai 2, Erfan Aref-Eshghi 3 and Bekim Sadikovic 1,2,4,* 1 Department of Pathology and Laboratory Medicine, Western University, London, ON N6A 3K7, Canada; [email protected] 2 Molecular Genetics Laboratory, Molecular Diagnostics Division, London Health Sciences Centre, London, ON N6A 5W9, Canada; [email protected] 3 Division of Genomic Diagnostics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; [email protected] 4 Schulich School of Medicine and Dentistry, Western University, London, ON N6A 5C1, Canada * Correspondence: [email protected] Received: 22 November 2020; Accepted: 4 December 2020; Published: 6 December 2020 Abstract: Mendelian neurodevelopmental disorders customarily present with complex and overlapping symptoms, complicating the clinical diagnosis. Individuals with a growing number of the so-called rare disorders exhibit unique, disorder-specific DNA methylation patterns, consequent to the underlying gene defects. Besides providing insights to the pathophysiology and molecular biology of these disorders, we can use these epigenetic patterns as functional biomarkers for the screening and diagnosis of these conditions. This review summarizes our current understanding of DNA methylation episignatures in rare disorders and describes the underlying technology and analytical approaches. We discuss the computational parameters, including statistical and machine learning methods, used for the screening and classification of genetic variants of uncertain clinical significance. Describing the rationale and principles applied to the specific computational models that are used to develop and adapt the DNA methylation episignatures for the diagnosis of rare disorders, we highlight the opportunities and challenges in this emerging branch of diagnostic medicine. -

Regional Genetic Consultation Service
Regional Genetic Consultation Service Contract 5889BD01 July 1, 2018 through June 30, 2019 Congenital & Inherited Disorders Division of Health Promotion & Chronic Disease Prevention, Phone: 1-800-383-3826 http://idph.iowa.gov/genetics Regional Genetic Consultation Service: A contract established by IDPH in the Division of Health Promotion & Chronic Disease Prevention, Contract # 5888BD01 Administrative Code, Chapter 4:641-4.5(136A) Iowa Department of Public Health Advancing Health Through the Generations Kimberly Reynolds Adam Gregg Gerd Clabaugh Governor Lt. Governor Director Authorized State Official for this contract: Brenda Dobson, Director, Division of Health Promotion and Chronic Disease Prevention (515) 281-7769 University of Iowa Stead Family Department of Pediatrics (319) 356-2675 Hatem El-Shanti, MD, Division Director John A. Bernat, MD, PhD, Clinical Director Hannah Bombei, MS, CGC, Program Coordinator Page | 2 Congenital & Inherited Disorders Division of Health Promotion & Chronic Disease Prevention, Phone: 800-383-3826 http://idph.iowa.gov/genetics In collaboration with the Iowa Department of Public Health & The Stead Family Department of Pediatrics Division of Medical Genetics & Genomics University of Iowa Stead Family Children’s Hospital & Clinical Outreach Services University of Iowa Hospitals and Clinics Page | 3 What is the Regional Genetic Consultation Service? Iowa Administrative Code 641—4.5(136A) Regional Genetic Consultation Service (RGCS). This program provides comprehensive genetic and genomic services statewide through outreach clinics. 4.5(1) Provision of comprehensive genetic and genomic services. The department shall contract with the Division of Medical Genetics & Genomics within the Stead Family Department of Pediatrics at the University of Iowa to provide genetic and genomic health care and education outreach services for individuals and families within Iowa. -

Abstracts Books 2014
TWENTY-FIFTH EUROPEAN MEETING ON DYSMORPHOLOGY 10 – 12 SEPTEMBER 2014 25th EUROPEAN MEETING ON DYSMORPHOLOGY LE BISCHENBERG WEDNESDAY 10th SEPTEMBER 5 p.m. to 7.30 p.m. Registration 7.30 p.m. to 8.30 p.m. Welcome reception 8.30 p.m. Dinner 9.30 p.m. Unknown THURSDAY 11th SEPTEMBER 8.15 a.m. Opening address 8.30 a.m. to 1.00 p.m. First session 1.00 p.m. Lunch 2.30 p.m. to 7.00 p.m. Second and third sessions 8.00 p.m. Dinner 9.00 p.m. to 11.00 p.m. Unknown FRIDAY 12th SEPTEMBER 8.30 a.m. to 1.00 p.m. Fourth and fifth sessions 1.00 p.m. Lunch 2.30 p.m. to 6.00 p.m. Sixth and seventh sessions 7.30 p.m. Dinner SATURDAY 13th SEPTEMBER Breakfast – Departure Note: This program is tentative and may be modified. WEDNESDAY 10th SEPTEMBER 9.30 UNKNOWN SESSION Chair: FRYNS J.P. H. VAN ESCH Case 1 H. VAN ESCH Case 2 H. JILANI, S. HIZEM, I. REJEB, F. DZIRI AND L. BEN JEMAA Developmental delay, dysmorphic features and hirsutism in a Tunisian girl. Cornelia de Lange syndrome? L. VAN MALDERGEM, C. CABROL, B. LOEYS, M. SALEH, Y. BERNARD, L. CHAMARD AND J. PIARD Megalophthalmos and thoracic great vessels aneurisms E. SCHAEFER AND Y. HERENGER An unknown diagnosis associating facial dysmorphism, developmental delay, posterior urethral valves and severe constipation P. RUMP AND T. VAN ESSEN What’s in the name G. MUBUNGU, A. LUMAKA, K.