The Islands of Macaronesia Almost Entirely on Non-Islanders, This Situation Has Now Changed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Campanulaceae) Based on ITS and Tranl-F Sequence Data: Implications for a Reclassification
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of the Western Cape Research Repository Cupido, C. N. et al. (2013). Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and tranL-F sequence data: implications for a reclassification. Systematic Botany, 38(2): 523 – 535 http:// doi.org/10.1600/036364413X666714 dx. Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and trnL-F sequence data: implications for a reclassification Christopher N. Cupido , Jessica M. Prebble , and William M. M. Eddie Abstract The Campanulaceae: Wahlenbergioideae currently comprises 15 genera, one of which, Wahlenbergia, is widespread over the southern continents. Southern Africa is the region with maximum wahlenbergioid diversity with 12 genera and approximately 252 species. A second center is Australasia with 38 Wahlenbergia species. This study used a broad sample of wahlenbergioid diversity from South Africa, Australia, and New Zealand to reconstruct a phylogeny based on chloroplast trnL-F and nuclear ITS sequences. Data were analyzed separately and in combination using parsimony and Bayesian methods. The results suggest that for the wahlenbergioids to be monophyletic Wahlenbergia hederacea has to be excluded and that none of the South African, Australian or New Zealand lineages are strictly monophyletic. There are five species assemblages that are in some disagreement with current classification in the family. Wahlenbergia, Prismatocarpus and Roella are shown to be non-monophyletic and implications for a reclassification are presented. Careful consideration of morphological characters is suggested before the adjustment of generic circumscriptions can be accomplished. Recent family-wide molecular phylogenetic studies have supported the view that the Campanulaceae s.s. -

Compositional Variability of Pleistocene Land Snail Assemblages Preserved in a Cinder Cone
Compositional variability of Pleistocene land snail assemblages preserved in a cinder cone volcano from Tenerife, Canary Islands A thesis submitted to the graduate school of the University of Cincinnati in partial fulfillment of the requirements for the degree of Master of Science In the Department of Geology of the College of Arts and Sciences by Elizabeth M. Bullard B.S., Muskingum University, 2012 July 2016 Advisors: Dr. Yurena Yanes Dr. Arnold I. Miller Committee Member: Dr. Joshua Miller i Abstract Fossil assemblage faunal compositions may vary through space and time in response to climatic and/or taphonomic factors, but these relationships can be difficult to diagnose and disentangle. Here, we investigate how to disentangle climatic and taphonomic signals of a land- snail-rich volcanic scoria sequence to asses if it was influenced by taphonomic bias, climate change, or both, using a multifaceted approach, combining taphonomic, ecological, body-size, and stable-isotope data. Fossil assemblages were sampled from two beds (Units A and B) in a Pleistocene cinder cone volcano of southern Tenerife (Canary Islands), dated to the glacial interval MIS 8 (~299-302 ka). The two units differed in taphonomy, species composition, and abundance distributions. The upper unit, B (6 species), showed higher snail diversity and shell concentration and lower taphonomic alteration than the lower unit, A (3 species). Furthermore, larger bodied species (length>10mm) dominated Unit A and were better preserved than smaller species (length<10mm), whereas smaller individuals were more abundant (and better preserved) at Unit B. These differences were likely impacted by physical differences in the matrices surrounding the fossils. -

Spatial Predictive Distribution Modelling of Madeira's Endemic
DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dinarte Nuno Freitas Teixeira 2009 REGIÃO AUTÓNOMA DA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA MADEIRA FSE DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Professor Doutor José Paulo Sousa (Universidade de Coimbra) e do Professor Doutor José Manuel Jesus (Universidade da Madeira). Dinarte Nuno Freitas Teixeir a 2009 O presente trabalho foi financiado pelo Centro de Ciência e Tecnologia da Madeira (CITMA), através da bolsa de Mestrado FSE BM I/2008 – 531, ao abrigo do Programa Operacional de Valorização do Potencial Humano e Coesão Social da RAM (RUMOS). REGIÃO AUTÓNOMA DA MADEIRA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA FSE À Susana AGRADECIMENTOS Esta tese é o resultado de um trabalho conjunto para o qual muitos contribuíram e aos quais desejo reconhecer e deixar o meu agradecimento. Ao professor Doutor José Paulo Sousa, meu orientador, pela indispensável ajuda, paciência e orientação científica. Ao professor Doutor José Manuel Jesus, meu orientador, pela amizade e apoio desde os primeiros momentos. Pelo seu empenho, conselhos transmitidos, chamadas à razão e orientação científica o meu muito obrigado. Ao Doutor Pedro Cardoso, meu orientador e a quem muito devo, pelo constante acompanhamento e disponibilidade, amizade e orientação científica. Por tudo o que me ensinou, pela motivação e animo que sempre me transmitiu, e, acima de tudo, pela manutenção da objectividade do trabalho. -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

Terrestrial Arthropods of Macaronesia
TERRESTRIAL ARTHROPODS OF MACARONESIA Biodiversity, Ecology and Evolution Title Terrestrial Artrhropods of Macaronesia - Biodiversity, Ecology and Evolution 1st edition, 2010 Editors Artur R. M. Serrano, Paulo A. V. Borges, Mário Boieiro and Pedro Oromí Sociedade Portuguesa de Entomologia Finantial support provided by Fundação para a Ciência e a Tecnologia, Portugal Project PDCT/BIA-BDE/59202/2004 Cover design by Security Print Cover photographs (and author): Desertas Islands (Photo by SPNM), Alloxantha fulva (Photo by P. Oromí) Misumena spinifera (Photo by P. Oromí), Guanchia uxoris (Photo by P. Oromí), Acrostira euphorbiae (Photo by P. Oromí), Dolichoiulus xylomistax (Photo by P. Oromí), Longitarsus isoplexidis (Photo by A. Serrano), Backcover photographs (and author): Selvagem Grande - Selvagens (Photo by SPNM), Turinyphia cavernicola (Photo by P. Borges), Herpisticus eremita (Photo by P. Oromí), Pseudoyersinia pilipes (Photo by P. Oromí), Hogna schmitzi (Photo by P. Oromí), Ischnura hastata (Photo by A. Cordero Ribera), Domene vulcanica (Photo by P. Oromí) Printed by Security Print - Sociedade de Indústria Gráfica, Lda. ISBN: 978-972-97241-2-1 Depósito Legal: XXX $POUFOUT __________________________________________________________________________________________ Preface by Antonio Machado INTRODUCTION Chapter 1 - The islands of Macaronesia, 1 J.M. Fernández-Palacios SECTION: BIODIVERSITY and CONSERVATION Chapter 2 - The provisional status of terrestrial arthropod inventories in the Macaronesian islands, 33 Jorge M. Lobo & Paulo A.V. Borges Chapter 3 - The Macaronesian province: patterns of species richness and endemism of arthropods, 49 Kostas A. Triantis, Paulo A.V. Borges, Joaquín Hortal & Robert J. Whittaker Chapter 4 - Patterns of Alpha and Beta Diversity of Epigean Arthropods at Contrasting Land-Uses of an Oceanic Island (Terceira, Azores), 73 Pedro Cardoso, Clara Gaspar, Francisco Dinis & Paulo A.V. -
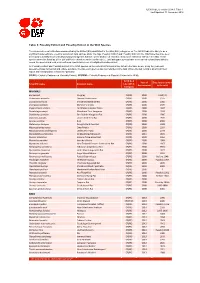
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -

COSSMANNIANA Bulletin Du Groupe D'étude Et De Recherche Macrofaune Cénozoïque
COSSMANNIANA Bulletin du Groupe d'Étude et de Recherche Macrofaune Cénozoïque Tome 3, numéro 4 Décembre 1995 ISSN 1157-4402 GROUPE D'ÉTUDE ET DE RECHERCHE MACROFAUNE CÉNOZOïQUE "Maisonpour tous" 26, rue Gérard Philippe 94120 FONTENAY-SOUS-SOIS Président . .. ... Jacques PONS Secrétaire .. PierreLOZOUET Trésorier . .. .. Philippe MAESTRATI Dessins de couverture : Jacques LERENARD Maquette et Édition: Jacques LERENARD [eau-Michel PACAUD Couverture: Campanile (Campanilopa) giganteum, d'après la figure 137-45 de l'tconographie (grossissement 3/8); et individu bréphiqu e (hauteur totale : 2 mm), muni de son périostracum et de sa protoconque (coll. LeRenard) . COSSMANNIANA, Paris, 3 (4), Décembre 1995, pp. 133-150, sans fig. ISSN: 1157-4402 . RÉVISION DES MOLLUSQUES PALÉOGÈNES DU BASSIN DE PARIS III - CHRONOLOGIE DES CRÉATEURS DE RÉFÉRENCES PRIMAIRES par Jacques ·LERENARD Laboratoire de Biologiedes Invertébrés Marins et Malacologie, Muséum.National d'Histoire Naturelle, 55,rue de Buffon- 75005 Paris - FRANCE RÉSUMÉ - La liste des 437 publications dans lesquelles ont été introduites des références primaires figurant dans la partie II (LERENARD & PACAUD, 1995, pp. 65-132) est donnée. Il s'agit de la première liste de l'ensemble des publications concernant des nouveaux noms ou des nouvelles espèces paléogènes de Mollusques du bassin de Paris. TITLE - Revision of the Paris Basin Paleogene MoIlusca. III: Chronological Iist of the authors of primary references. ABSTRACT - The 437 publications, in which the primary references cited in part II (LERENARD & PACAUD, 1995, pp. 65-132) have been introduced, are given. This constitutes the first list of aIl the publications that are conceming new species or new names of Paris Basin Paleogene Molluscan species. -

Canariella Planaria
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T156901A5014481 Canariella planaria Assessment by: Groh, K. & Neubert, E. View on www.iucnredlist.org Citation: Groh, K. & Neubert, E. 2013. Canariella planaria. The IUCN Red List of Threatened Species 2013: e.T156901A5014481. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T156901A5014481.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Hygromiidae Taxon Name: Canariella planaria (Lamarck, 1822) Assessment Information Red List Category & Criteria: Least Concern ver 3.1 Year Published: 2013 Date Assessed: March 24, 2011 Justification: This species is endemic to northeastern coastal area of the island of Tenerife. -

Malacologica
FOLIA Folia Malacol. 24(3): 111–177 MALACOLOGICA ISSN 1506-7629 The Association of Polish Malacologists Faculty of Biology, Adam Mickiewicz University Bogucki Wydawnictwo Naukowe Poznań, September 2016 http://dx.doi.org/10.12657/folmal.024.008 PATTERNS OF SPATIO-TEMPORAL VARIATION IN LAND SNAILS: A MULTI-SCALE APPROACH SERGEY S. KRAMARENKO Mykolaiv National Agrarian University, Paryzka Komuna St. 9, Mykolaiv, 54020, Ukraine (e-mail: [email protected]) ABSTRACT: Mechanisms which govern patterns of intra-specific vatiation in land snails were traced within areas of different size, using Brephulopsis cylindrica (Menke), Chondrula tridens (O. F. Müller), Xeropicta derbentina (Krynicki), X. krynickii (Krynicki), Cepaea vindobonensis (Férussac) and Helix albescens Rossmässler as examples. Morphometric shell variation, colour and banding pattern polymorphism as well as genetic polymorphism (allozymes and RAPD markers) were studied. The results and literature data were analysed in an attempt to link patterns to processes, with the following conclusions. Formation of patterns of intra- specific variation (initial processes of microevolution) takes different course at three different spatial scales. At micro-geographical scale the dominant role is played by eco-demographic characteristics of the species in the context of fluctuating environmental factors. At meso-geographical scale a special part is played by stochastic population-genetic processes. At macro-geographical scale more or less distinct clinal patterns are associated with basic macroclimatic -

Na História Da Paleontologia
Bol. R. Soc. Esp. Hist. Nat., 115, 2020: pediente de paginación Os topónimos das ilhas atlânticas da Comunidade dos Países de Língua Portuguesa (CPLP) na história da Paleontologia Los topónimos de las islas atlánticas de la Comunidad de Países de Lengua Portuguesa (CPLP) en la historia de la Paleontología The toponyms of the Atlantic islands from the Community of Portuguese Speaking Counties (CPLP) in the history of Palaeontology Rogério B. Rocha (†)1, Pedro M. Callapez2,3, José C. Kullberg1 & Paulo S. Caetano1 1. Universidade NOVA de Lisboa, GeoBioTec - GeoBiociências, Geotecnologias e Geoengenharias, Faculdade de Ciências e Tecnologia, Departamento de Ciências da Terra, Quinta da Torre, P-2825 516 Caparica, Portugal. [email protected], [email protected] 2. Universidade de Coimbra, CITEUC - Centro de Investigação da Terra e do Espaço da Universidade de Coimbra, Faculdade de Ciências e Tecnologia, Departamento de Ciências da Terra, Rua Sílvio Lima, P-3030 790 Coimbra, Portugal. [email protected] 3. PALEOIBERICA - Grupo de Investigación Consolidado de la Universidad de Alcalá (COD-824), Departamento de Geología, Geografía y Medio Ambiente, 28805 Alcalá de Henares, España Recibido: 8 de abril de 2020. Aceptado: 7 de abril de 2021. Publicado electrónicamente: 26 de abril de 2021. PALAVRAS CHAVE: Nomenclatura paleontológica, Topónimo, História da Paleontologia, Açores e Madeira, Cabo Verde, Macaronésia. PALABRAS CLAVE: Nomenclatura paleontológica, Topónimo, Historia de la Paleontología, Azores y Madeira, Cabo Verde, Macaronesia. KEYWORDS: Palaeontological nomenclature, Toponym, History of Palaeontology, Azores and Madeira, Cape Verde, Macaronesia. RESUMO O recurso a topónimos locais é de uso frequente na descrição científica de novas espécies. Numa perspetiva histórica da taxonomia paleontológica, estes enriquecem substancialmente a nomenclatura existente e permitem contextualizações geográficas mais efetivas. -
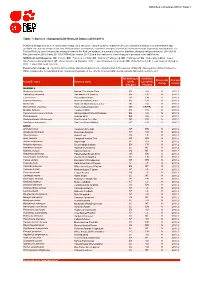
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

Canariella Eutropis (Shuttleworth, 1860) EN
Libro Rojo de los Invertebrados de España Canariella eutropis (Shuttleworth, 1860) EN Categoría UICN: En peligro Criterio UICN: A3ce; B2ab(iii) Nombre Vulgar: Tipo: Mollusca Clase: Gastropoda Orden: Pulmonata Familia: Hygromiidae Área de distribución trucción, concretamente una autopista y nume- Endémica de la península de Jandía, en la isla de rosos alojamientos hoteleros. Fuerteventura. Ocupa un área de alrededor de 10 km2, incluyendo los acantilados del lado norte de Medidas de conservación los montes de Jandía y la plataforma costera sobre Protección de su hábitat, estableciendo una Re- la que se elevan. Antiguamente ocupaba un área serva Natural Integral en una parte de su área de muy superior, de unos 30 km2 (este dato se cono- distribución. Bastaría con impedir la entrada de ce gracias al registro fósil; datos no publicados). ganado (tanto cabras como ovejas) en un área de Localidad tipo: Fuerteventura (Canarias). unos 4 km2 (aproximadamente, 8 km de longitud por 0,5 km de anchura). Por la pequeña superfi- Hábitat y Biología cie, no afectaría prácticamente a los intereses eco- Vegetación del piso basal (xérico) y vegetación nómicos de los habitantes de la zona, haciendo potencial del bosque termófilo (hoy degradada) falta tan sólo vallarla para evitar la entrada de ru- de los acantilados orientados al norte de los mon- miantes. La entrada de personas queda autoex- tes de Jandía. cluida por tratarse en su mayor parte de precipi- cios. Sus límites por el lado sur estarían formados Factores de amenaza por la dorsal de la cordillera de Jandía, entre el – Sobre la población: El área pequeña que ocupa, Morro de la Burra en su extremo oriental y el Pico en los montes de Jandía.