Compositional Variability of Pleistocene Land Snail Assemblages Preserved in a Cinder Cone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Top100amea.Pdf
Editores / Editores José Luis Martín Esquivel Manuel Arechavaleta Hernández Paulo A. V. Borges Bernardo F. Faria Edición y financiación / Ediçao e financiamento INTERREG III-B BIONATURA Dirección General del Medio Natural, Gobierno de Canarias ARENA, Governo Regional dos Açores Direcção Regional do Ambiente, Governo Regional da Madeira Modo de citar la obra / Modo de fazer mençao a obra Cuando se hace referencia a la obra / Quando fazer refêrencia a obra: MARTÍN, J. L., M. ARECHAVALETA, P. A. V. BORGES & B. FARIA (eds.). 2008. Top 100. Las 100 especies amenazadas prio- ritarias de gestión en la región europea biogeográfica de la Macaronesia. Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias. 500 pp. Cuando se hace referencia a un capítulo de la obra / Quando fazer refêrencia a um capítulo da obra: FARIA, B. F., C. ABREU, A. F. AGUIAR, J. AUGUSTO, R. JARDIM, C. LOBO, P. OLIVEIRA & D. TEIXEIRA. 2008. La perspectiva archipe- lágica: Madeira. En: MARTÍN, J. L., M. ARECHAVALETA, P. A. V. BORGES & B. FARIA (eds.). Top 100. Las 100 especies ame- nazadas prioritarias de gestión en la región europea biogeográfica de la Macaronesia. Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias. pp.: 109-128. Cuando se hace referencia a una ficha de especie /Quando fazer refêrencia a uma ficha de espécie: MARTINS, M., M. MOURA & L. SILVA. 2008. Azorina vidalii (H.C. Watson) Feer. En: MARTÍN, J. L., M. ARECHAVALETA, P. A. V. BORGES & B. FARIA (eds.). Top 100. Las 100 especies amenazadas prioritarias de gestión en la región europea biogeográfica de la Macaronesia. Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Ca- narias. -

Revision of the Systematic Position of Lindbergia Garganoensis
Revision of the systematic position of Lindbergia garganoensis Gittenberger & Eikenboom, 2006, with reassignment to Vitrea Fitzinger, 1833 (Gastropoda, Eupulmonata, Pristilomatidae) Gianbattista Nardi Via Boschette 8A, 25064 Gussago (Brescia), Italy; [email protected] [corresponding author] Antonio Braccia Via Ischia 19, 25100 Brescia, Italy; [email protected] Simone Cianfanelli Museum System of University of Florence, Zoological Section “La Specola”, Via Romana 17, 50125 Firenze, Italy; [email protected] & Marco Bodon c/o Museum System of University of Florence, Zoological Section “La Specola”, Via Romana 17, 50125 Firenze, Italy; [email protected] Nardi, G., Braccia, A., Cianfanelli, S. & Bo- INTRODUCTION don, M., 2019. Revision of the systematic position of Lindbergia garganoensis Gittenberger & Eiken- Lindbergia garganoensis Gittenberger & Eikenboom, 2006 boom, 2006, with reassignment to Vitrea Fitzinger, is the first species of the genus, Lindbergia Riedel, 1959 to 1833 (Gastropoda, Eupulmonata, Pristilomatidae). be discovered in Italy. The genus Lindbergia encompasses – Basteria 83 (1-3): 19-28. Leiden. Published 6 April 2019 about ten different species, endemic to the Greek mainland, Crete, the Cycladic islands, Dodecanese islands, northern Aegean islands, and southern Turkey (Riedel, 1992, 1995, 2000; Welter-Schultes, 2012; Bank & Neubert, 2017). Due to Lindbergia garganoensis Gittenberger & Eikenboom, 2006, lack of anatomical data, some of these species remain ge- a taxon with mainly a south-Balkan distribution, is the only nerically questionable. Up to now, L. garganoensis was only Italian species assigned to the genus Lindbergia Riedel, 1959. known by the presence of very fine spiral striae on the tel- The assignment to this genus, as documented by the pecu- eoconch and by the general shape of its shell. -

The Field Museum 2002 Annual Report to the Board of Trustees Academic Affairs
THE FIELD MUSEUM 2002 ANNUAL REPORT TO THE BOARD OF TRUSTEES ACADEMIC AFFAIRS Office of Academic Affairs, The Field Museum 1400 South Lake Shore Drive Chicago, IL 60605-2496 USA Phone (312) 665-7811 Fax (312) 665-7806 WWW address: http://www.fieldmuseum.org - This Report Printed on Recycled Paper - -1- Revised May 2003 -2- CONTENTS 2002 Annual Report....................................................................................................................................................3 Collections and Research Committee.....................................................................................................................12 Academic Affairs Staff List......................................................................................................................................13 Publications, 2002 .....................................................................................................................................................19 Active Grants, 2002...................................................................................................................................................38 Conferences, Symposia, Workshops and Invited Lectures, 2002 .......................................................................46 Museum and Public Service, 2002 ..........................................................................................................................55 Fieldwork and Research Travel, 2002 ....................................................................................................................65 -

Diplom-Biologe KLAUS GROH Malakozoologe Und Naturschützer – 65 Jahre
53 Mitt. dtsch. malakozool. Ges. 94 53 – 70 Frankfurt a. M., November 2015 Diplom-Biologe KLAUS GROH Malakozoologe und Naturschützer – 65 Jahre CARSTEN RENKER & JÜRGEN H. JUNGBLUTH th Abstract: The 65 birthday of KLAUS GROH is a good occasion to give a retrospect of his life and hitherto existing achievement. Beside his vita we summarize his malacological work, give an overview about the projects for the protection of species, have a look on his tremendous impetus for the worldwide distribution of malacological knowledge by the establishment of the CHRISTA HEMMEN-Verlag, later ConchBooks, as publishing house, book trader and antiquarian. Last but not least we give a summary of his scientific achievements culminating in 206 publications and containing descriptions of up to now 42 specific taxa. Keywords: KLAUS GROH, biography, bibliography, malacology, freshwater mussels, Hesse, Rhineland- Palatinate, Luxembourg Zusammenfassung: Der 65. Geburtstag von KLAUS GROH wird zum Anlass genommen einen Rückblick auf sein bisheriges Leben und Wirken zu geben. Neben der Vita werden vor allem seine malakologische Arbeit und sein ehrenamtliches Engagement in zahlreichen malakologischen Verbänden und Naturschutzvereinen betrachtet. KLAUS GROH nahm außerdem einen enormen Einfluss auf die weltweite Verbreitung malako- logischen Wissens durch die Gründung des CHRISTA HEMMEN-Verlags, später ConchBooks, als Verlagshaus, Buchhandlung und Antiquariat. Schließlich gilt es seine wissenschaftlichen Verdienste zu würdigen, die in 206 Publikationen und Neubeschreibungen 42 spezifischer Taxa kulminieren. Vita Schulzeit Am 22. Mai 1949 wurde KLAUS GROH in Darmstadt als Sohn des Bauschlossers HELMUT GROH und seiner Ehefrau ANNELIESE, geb. FEDERLEIN geboren. Er besuchte die Volksschulen in Langen/Hessen und Kirchheim unter Teck/Baden-Württemberg (1955-1959), es folgte der Besuch der Realschule in Langen/Hessen (1959-1965), dort schloss er auch seine Schulzeit mit der „Mittleren Reife“ ab. -

Description of a Representative of the Genus Hemicycla Canary Islands
BASTERIA, 70: 53-56, 2006 of discovered extinct of the Description a newly representative genus Hemicycla Swainson, 1840 (Gastropoda, Pulmonata, Helicidae) from La Gomera, Canary Islands T. Beck Institut fur Palaontologie der Universitat Erlangen, Loewenichstrasse 28, D 91054 Erlangen, Germany; [email protected] & W. Rähle Engelfriedshalde 102, D-72076 Tubingen, Germany; [email protected] A fossil of the from La Gomera is described. It found in new species genus Hemicycla was Quaternary deposits near Hermigua in the northeast of the island. The shells are considerably larger than those of all other Hemicycla species from the Canary Islands. Hemicycla montefortia- shows malleated shell whereas the shell surface smooth. na spec. nov. a strongly sculpture is Key words: Gastropoda, Pulmonata,Helicidae, Hemicycla, taxonomy, new species, Quaternary, La Gomera, Canary Islands, Spain. At the slopes of numerous barrancos on the island of La Gomera Quaternary depo- sits are exposed. They are deeply cut by the erosive activities of the actual brooks. According to Groh et al. (1996) these deposits have to be interpreted as remnants of the bottoms of ancient, presumably Pleistocene valleys. At many localities shells of land snails can be found in such deposits. In some out- that still the island with crops species are living on are co-occuring supposed extinct spe- cies only known from fossil records. In the checklist Bank, Groh & 12 of the published by Ripken (2002) species genus Hemicycla Swainson, 1840, are mentioned to occur on La Gomera. Five taxa [J[H. gomerensis (Mousson, 1872), H. hedybia (Mabille, 1882), H. paivanopsis (Mabille, 1882), H. -

Fauna of New Zealand Ko Te Aitanga Pepeke O Aotearoa
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Canariella Planaria
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T156901A5014481 Canariella planaria Assessment by: Groh, K. & Neubert, E. View on www.iucnredlist.org Citation: Groh, K. & Neubert, E. 2013. Canariella planaria. The IUCN Red List of Threatened Species 2013: e.T156901A5014481. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T156901A5014481.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Hygromiidae Taxon Name: Canariella planaria (Lamarck, 1822) Assessment Information Red List Category & Criteria: Least Concern ver 3.1 Year Published: 2013 Date Assessed: March 24, 2011 Justification: This species is endemic to northeastern coastal area of the island of Tenerife. -

Hemicycla (Adiverticula) Diegoi (Gastropoda: Pulmonata: Helicidae
Zootaxa 2757: 29–46 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Hemicycla (Adiverticula) diegoi (Gastropoda: Pulmonata: Helicidae), a new species from Tenerife, Canary Islands, with a phylogenetic analysis of conchologically similar species in the genus Hemicycla Swainson, 1840 MARCO T. NEIBER1,5, RICARDO VEGA-LUZ2, RODOLFO VEGA-LUZ3 & STEFAN KOENEMANN4 1University of Veterinary Medicine Hannover, Institute for Animal Ecology and Cell Biology, Bünteweg 17d, D-30559 Hannover, Ger- many. E-mail: [email protected] 2C/ Golf de El Candido 49, E-29018 Malaga, Spain. E-mail: [email protected] 3Ctra. Punta de Teno s/n., Teno Bajo, E-38480 Buenavista del Norte, Spain 4University of Siegen, Department of Biology and Didactics, Adolf-Reichwein-Str. 2, D-57068 Siegen, Germany. E-mail: koene- [email protected] 5 Corresponding author Abstract Hemicycla (Adiverticula) diegoi n. sp. from the westernmost part of the Teno massif above the Lighthouse at Punta de Teno, Tenerife, Canary Islands is described and compared to conchologically and anatomically similar species in the genus Hemicycla Swainson, 1840. The validity of the new species is corroborated by a phylogenetic analysis including several congeneric species, inter- and intraspecific genetic distances and a morphometric comparison by means of a discriminant function analysis. Hemicycla cf. paivanopsis (Mabille, 1882) and Hemicycla quadricincta quadricincta (Morelet, 1864) from La Gomera and Hemicycla berkeleii (R. T. Lowe, 1861) from Gran Canaria are tentatively placed in Hemicycla s. str. on the basis of a phylogenetic analysis. Furthermore, Eobania vermiculata (O. -

Biodiversity of the Hypersaline Urmia Lake National Park (NW Iran)
Diversity 2014, 6, 102-132; doi:10.3390/d6010102 OPEN ACCESS diversity ISSN 1424-2818 www.mdpi.com/journal/diversity Review Biodiversity of the Hypersaline Urmia Lake National Park (NW Iran) Alireza Asem 1,†,*, Amin Eimanifar 2,†,*, Morteza Djamali 3, Patricio De los Rios 4 and Michael Wink 2 1 Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China 2 Institute of Pharmacy and Molecular Biotechnology (IPMB), Heidelberg University, Im Neuenheimer Feld 364, Heidelberg D-69120, Germany; E-Mail: [email protected] 3 Institut Méditerranéen d’Ecologie et de Paléoécologie UMR 6116 du CNRS-Europôle Méditerranéen de l’Arbois-Pavillon Villemin-BP 80, Aix-en-Provence Cedex 04 13545, France; E-Mail: [email protected] 4 Environmental Sciences School, Natural Resources Faculty, Catholic University of Temuco, Casilla 15-D, Temuco 4780000, Chile; E-Mail: [email protected] † These authors contributed equally to this work. * Authors to whom correspondence should be addressed; E-Mails: [email protected] (A.A.); [email protected] (A.E.); Tel.: +86-150-6624-4312 (A.A.); Fax: +86-532-8203-2216 (A.A.); Tel.: +49-6221-544-880 (A.E.); Fax: +49-6221-544-884 (A.E.). Received: 3 December 2013; in revised form: 13 January 2014 / Accepted: 27 January 2014 / Published: 10 February 2014 Abstract: Urmia Lake, with a surface area between 4000 to 6000 km2, is a hypersaline lake located in northwest Iran. It is the saltiest large lake in the world that supports life. Urmia Lake National Park is the home of an almost endemic crustacean species known as the brine shrimp, Artemia urmiana. -
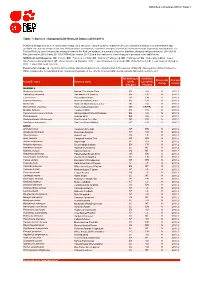
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

Una Nueva Especie De Tambja Burn, 1962 (Mollusca, Nudibranchia)
Bull. Mus. natn. Hist. nal.. Paris. 4' sér., 10, 1988, section A, n° 2 : 301-307. Una nueva especie de Tambja Burn, 1962 (Mollusca, Nudibranchia) por José Carlos GARCÍA GÓMEZ y Jesús ORTEA Resumen. — Una nueva especie de Tambja Burn, 1962 del estrecho de Gibraltar, Tambja ceutae n. sp. se distingue de todas las restantes especies del género por la presencia de pequeñas papilas cónicas bordeando el noto, y por su próstata netamente diferenciada del canal deferente. Su notable coloración, verde azulada con bandas longitudinales amarillas, hace que sea uno de los Nudibranquios mas espectaculares del área europea, donde no puede ser confundido con ningún otro. Resume. — Une nouvelle espèce de Tambja Burn, 1962 du détroit de Gibraltar, Tambja ceutae n. sp., se distingue de toutes les autres espèces du genre par la présence de petites papilles coniques bordant le notum, et par sa prostate nettement différenciée du canal déférent. Sa couleur remarquable, bleu vert avec des bandes longitudinales jaunes, en fait un des Nudibranches les plus spectaculaires du domaine européen, où il ne peut être confondu avec aucun autre. J. C. GARCÍA GÓMEZ, Dep. Zoología, Fac. Biología, Univ. Sevilla, España. ¡. ORTEA, Dep. Zoología, Fac. Biología, Univ. Oviedo, España. INTRODUCCIÓN En un trabajo anterior, ORTEA y GARCÍA (1986), describíamos una nueva especie de Tambja Burn, capturada en las islas de Cabo Verde, que suponía la primera referencia en el Atlántico Nordeste de un género de Gymnodoridos sin procesos frontales cuya rádula se caracteriza por tener una muesca en la zona media del diente raquídeo y un primer diente lateral bífido. -

Canariella Eutropis (Shuttleworth, 1860) EN
Libro Rojo de los Invertebrados de España Canariella eutropis (Shuttleworth, 1860) EN Categoría UICN: En peligro Criterio UICN: A3ce; B2ab(iii) Nombre Vulgar: Tipo: Mollusca Clase: Gastropoda Orden: Pulmonata Familia: Hygromiidae Área de distribución trucción, concretamente una autopista y nume- Endémica de la península de Jandía, en la isla de rosos alojamientos hoteleros. Fuerteventura. Ocupa un área de alrededor de 10 km2, incluyendo los acantilados del lado norte de Medidas de conservación los montes de Jandía y la plataforma costera sobre Protección de su hábitat, estableciendo una Re- la que se elevan. Antiguamente ocupaba un área serva Natural Integral en una parte de su área de muy superior, de unos 30 km2 (este dato se cono- distribución. Bastaría con impedir la entrada de ce gracias al registro fósil; datos no publicados). ganado (tanto cabras como ovejas) en un área de Localidad tipo: Fuerteventura (Canarias). unos 4 km2 (aproximadamente, 8 km de longitud por 0,5 km de anchura). Por la pequeña superfi- Hábitat y Biología cie, no afectaría prácticamente a los intereses eco- Vegetación del piso basal (xérico) y vegetación nómicos de los habitantes de la zona, haciendo potencial del bosque termófilo (hoy degradada) falta tan sólo vallarla para evitar la entrada de ru- de los acantilados orientados al norte de los mon- miantes. La entrada de personas queda autoex- tes de Jandía. cluida por tratarse en su mayor parte de precipi- cios. Sus límites por el lado sur estarían formados Factores de amenaza por la dorsal de la cordillera de Jandía, entre el – Sobre la población: El área pequeña que ocupa, Morro de la Burra en su extremo oriental y el Pico en los montes de Jandía.