Toxicological Profile for Chlordane Was Released in 1994, and an Addendum to This Profile Was Released in 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Union and Journal
m I BE TRUE, AND FAITHFUL, AND VALIANT FOR THE PUBLIC LIBERTIES IETOR. * VOLUME XXL BIDDEFORD, ME.. FRIDAY MORNING, AUGUST 25, 18B5. NUMBER 35. wm ■a- •' nnd inarnlml fur XOJI SWIPKRT TAXIS. He rerv felt with Mr Fessen- so and he Petroleum V. on the Political (Jo home, my friend, Spare L4nes. BERWICK ACADEMY, justly thai, transparent unassuming. Naiby Sarah heirs 173 SO den's und known at the Hit friend told ^ie that he was a Situation. the conflict. Tell jour ecntrai committia to SOTTU ■KR'WICK, MAINE. Cleave*, ot experience ability gacious. The eoldierecml! lira biacnit the "rook 0 F & 115 00 aruej Emery Co., head of the Finance Committee, his man of Ilia neighbor, to collect axpeneo tnunnj, and ! and Genral A Folaom, 376 00 accept- profound policy. The Cincinnati Commercial contain! the of agee." waa a Ferria and and FOUNDED 1791. Hobsoo, 1014 88 ance would go far towards re-establishing a whom I have referred, said he gnat Kernal Muebj Chap Fergu« Joseph ft keen document: ere wiee who make no useless crosses R Jo. 191 19 think Mowing •on nnd Bo and that Tlifj dan, jr., of Ho Mid to hira very thinker—that ho wu accustomed to Dick Turner and regard, Tfc» FALL TERM of thfa «UI eon. J King, heira of, 100 <13 feeling security. S/ixt's Rest, (which ia in the Suto ) for themselves to beer. " old off bat Dm Inrtrac. 143 75 the Lord has not de much on the affairs o( the nation. Soma* uv Noo noble hero (takn jour while I ■kik» on WEDNESDAY, Aug. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -
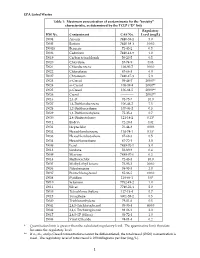
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

JOHN ANTHONY BARON CURRICULUM VITAE Education
JOHN ANTHONY BARON CURRICULUM VITAE Education: 1981-1982 Post-doctoral Milbank Scholar Department of Community Medicine and General Practice Oxford University 1980-1981 M.Sc. in Epidemiology London School of Hygiene and Tropical Medicine University of London 1972-1976 M.D. University of Michigan School of Medicine 1967-1969 M.S. in Mathematical Statistics (Ph.D. program interrupted by selective service) Stanford University 1963-1967 B.A. (Honors) in Intensive Mathematics Yale University Postdoctoral Clinical Training: 1979-80 Chief Medical Resident Dartmouth Medical School 1977-79 Residency in Internal Medicine Dartmouth Medical School 1976-77 Internship (Flexible, 8/12 Internal Medicine) University of Michigan Medical School Licensure and Certification: 1980 Board Certification - Internal Medicine 1979 New Hampshire Medical License #5911 1966 American Society of Actuaries - Examinations I and II Professional Experience: 2014-present Professor emeritus of Medicine and of Epidemiology Geisel School of Medicine at Dartmouth 2013-present Research Professor of Epidemiology Gillings School of Public Health University of North Carolina 2011-2013 Adjunct Professor of Epidemiology Gillings School of Global Health University of North Carolina 2010-present Professor of Medicine University of North Carolina School of Medicine 2010-present Visiting Professor Department of Clinical Epidemiology Aarhus University Denmark 1994-2003 Founding Section Chief, Biostatistics & Epidemiology Dept of Community and Family Medicine 1993-2002 Acting Director, then -

Health Consultation Chevron Chemical Co
- • ! t Health Consultation Chevron Chemical Co. (Ortho Division) Orlando, Orange County, Florida CERCLIS NO. FLD004064242 June 1995 Prepared by Environmental Toxicology The Florida Department of Health and Rehabilitative Services Under a Cooperative Agreement With Agency for Toxic Substances and Disease Registry U.S. Public Health Service Department of Health and Human Services - ' ' Health Consultation - Chevron Chemical June 1995 Background and Statement of Issues The purpose of this health consultation is to interpret the results of indoor air monitoring for pesticides in two trailers adjacent to the Chevron Chemical Superfund site in Orlando, Florida. During a March 9, 1995 public meeting, two nearby residents expressed concerns that the insides of their trailers were contaminated with pesticides. We agreed to test their trailers for pesticide contamination. The Chevron Chemical Co. (Ortho Division) Superfund site is a former pesticide formulation plant and truck repair facility in Orlando, Florida (Figures 1-3, Appendix A). Past waste disposal practices contaminated soil and ground water. Stormwater run-off carried pesticide-contaminated soil to the adjacent Armstrong Trailer Park. In 1992, the Chevron Chemical Company removed the on-site contaminated soil. In 1994, they removed the contaminated soil from the Armstrong Trailer Park. In a 1995 public health assessment (ATSDR 1995), we found the site was a public health hazard because some residents of the adjacent Armstrong Trailer Park may have unknowingly eaten small amounts of soil contaminated with the pesticide chlordane. As a result, we estimated those residents have a moderately increased risk of liver cancer. Since Chevron cleaned up the chlordane-contaminated soil at this trailer park, we estimated the remaining cancer risk from chlordane is insignificant. -

Chronology of Pesticides Used on National Park Service Collections
Conserve O Gram June 2001 Number 2/16 Chronology Of Pesticides Used On National Park Service Collections The history of National Park Service pesticide use publication). Synonyms and trade names were policy for museum collection objects is obtained from the Merck Index, notes from the documented in various publications including IPM Coordinator, and two Internet sites Field Manual for Museums (Burns), Manual for (<http://chemfinder.com> and <http:// Museums (Lewis), versions of the Museum www.cdpr.ca.gov/cgi-bin/epa>). Handbook, Part I, and two versions of the Integrated Pest Management Information Manual. Not all of the chemicals listed in the Other non-policy sources include Coleman's accompanying charts were marketed as pesticides. Manual for Small Museums, object treatment Some are fungicides and microbiocides. One, reports and notes from NPS staff, and notes from Lexol, is a leather preservative and consolidant. the Office of the Integrated Pest Management All of these are included here because records (IPM) Coordinator. indicate they were applied to objects as pesticides. The two accompanying charts list the types of The potential for pesticide residue remaining on pesticides that may have been used on National collection objects is very high. Objects with such Park Service collections along with some common residues pose a health risk to curatorial staff and synonyms and trade names. to the public who come into physical contact with them, unless proper precautions are taken. Dates shown in blue on the chart represent Additional information on health and safety issues published recommendations for the use of and protective measures can be found in the pesticides. -

Chlordane and Toxaphene Residues Following Cooking of Treated Channel Catfish Fillets
763 Journal of Food Protection, Vol. 63, No. 6, 2000, Pages 763±767 Copyright Q, International Association for Food Protection Chlordane and Toxaphene Residues following Cooking of Treated Channel Cat®sh Fillets C. R. SANTERRE,1* R. INGRAM,2 D. H. XU,3 G. W. LEWIS,4 AND L. G. LANE2 1Department of Foods and Nutrition, Purdue University, West Lafayette, Indiana 47907-1264; 2Mississippi State Chemical Laboratory, Mississippi State, Mississippi 39762; 3Department of Fisheries and Allied Aquacultures, Auburn University, Auburn, Alabama 36849; and 4Warnell School of Forest Resources, University of Georgia, Athens, Georgia 30602, USA MS 99-215: Received 28 July 1999/Accepted 17 December 1999 Downloaded from http://meridian.allenpress.com/jfp/article-pdf/63/6/763/1673844/0362-028x-63_6_763.pdf by guest on 27 September 2021 ABSTRACT The reduction in residues of chlordane and toxaphene following cooking (frying, baking, and smoking) of ®llets obtained from treated Channel cat®sh (Ictalurus punctatus) was determined. On average, cooking reduced moisture content by 17% and increased fat content by 28 to 274%. Frying reduced chlordane residues by 56 to 86% on a dry basis (db) or 84 to 92% on a percent fat basis (fb) when raw ®llets were compared to cooked ®llets. Baking and smoking reduced chlordane signi®cantly less (P , 0.05) than frying with reductions in residues of 12% and 9% (db) or 30% and 33% (fb), respectively. Frying reduced toxaphene residues by 40 to 49% (db) or 65 to 77% (fb), while baking and smoking reduced toxaphene by 35% and 24% (db) or 51% and 59% (fb), respectively. -

TOXICOLOGICAL PROFILE for CHLORDANE U.S. DEPARTMENT of HEALTH and HUMAN SERVICES Public Health Service Agency for Toxic Subs
TOXICOLOGICAL PROFILE FOR CHLORDANE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 1994 CHLORDANE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. CHLORDANE iii UPDATE STATEMENT A Toxicological Profile for Chlordane was released on December 1989. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 CHLORDANE vii CONTRIBUTORS CHEMICAL MANAGERS(S)/AUTHOR(S): Henry G. Abadin, M.S.P.H. ATSDR, Division of Toxicology, Atlanta, GA Ronald Baynes, D.V.M., M.S. Paul F. Goetchius, D.V.M. Syracuse Research Corporation, Syracuse, NY THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying endpoints. 3 . Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. 4 . Quality Assurance Review. The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problems in format or content, and establishes that Guidance has been followed. -

Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery
COMMON HOUSEHOLD HAZARDOUS WASTES CORROSIVES (ACIDS) Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery Acid Household Waxes Arsinic Compounds Copper Cleaners Isopropyl Alcohol Automotive Cleaners Etching Solutions Kerosene Bacterial Pipe Cleaners Ferric Chloride Lacquer Thiner Bordeaux Mix Hydrochloric Acid Lacquer Paint (unsolidified) Boric Acid Hydrofluoric Acid Linseed Oil Bug Removers Metal Cleaners Liquid Waxes Chlordane Muriatic Acid Liquid Sandpaper Chromium Navel Jelly Liquid Butane Copper Sulfate Phosphoric Acid Methanol DDT Pool Acid Naphtha Diazinon Sodium Bisulfate Oils (petroleum) Dimethylamine Salts Sulfuric Acid Organic Solvents Disinfectants Toilet Bowl Cleaners Paint Thinners Dog Repellent Paint Strippers Fertilizers CORROSIVES (BASES) Paraffin Oil Flea Spray/Powders Ammonia Perfume Fluorine Ammonia Based Cleaners Petroleum Distillers Fungicides Battery Terminal Cleaners Plastic Roof Cement Gopher Killer Caustic Soda Plastic Model Cement Hydrofluoric Acid Cess Pool Cleaners Polyurethane Paint (unsolidified) Insect Sprays Drain Cleaners Polyurethane Cement (unsolid.) Lead Compounds Household Cleaners Powe Steering Fluid Lice Powders Lime Primers Lindane Lye Roofing Cement Malathion Oven Cleaners Rug/Upholstery Cleaner Methylene Chloride Sodium Hydroxide Sealers Mole Killer Window Cleaners Shellac Thinner Moth Crystals Silicone Sprays Pesticides FLAMMABLES & COMBUSTIBLES Spot Remover/Dry Clean Fluids Pharmaceuticals Acetone Thinner Plant Food Adhesives Tile Cement Pruning Paint Aerosol Tire Black Pyethrins -

Table 1 Summary of Soil Analytical Methods
TABLE 1 SUMMARY OF SOIL ANALYTICAL METHODS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California No. of Analyzed Compound EPA Method Soil Samples Organochlorine Pesticides (OCPs) EPA Method 8081A 26 Total Lead EPA Method 6010B 30 Arsenic EPA Method 6010B 73 Page 1 of 1 TABLE 2 SUMMARY OF SOIL ANALYTICAL RESULTS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California Number. of Number of Analyzed Range of Analyzed Samples with Compounds Detections Samples Detections Organochlorine Pesticides 26 2 2-26 ug/kg a-chlordane 26 2 both 5 ug/kg d-chlordane 26 2 3-4 ug/kg total chlordane 26 2 11-26 ug/kg dieldrin 26 1 2 ug/kg Total Lead 30 29 1-53 mg/kg Arsenic 73 46 1-34 mg/kg mg/kg - milligrams per kilogram ug/kg - micrograms per kilogram mdl - method detection limit Page 1 of 1 TABLE 3 SUMMARY OF SOIL ANALYTICAL RESULTS PESTICIDES CRENSHAW HIGH SCHOOL 5010 11the Avenue Los Angeles, California Compound PB-2-6" PB-3-6" PB-5-6" PB-8-6" PB-12-6" PB-13-6" PB-18-6" EPA Method 8081A (ug/kg) Aldrin ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-BHC (Lindane) ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 beta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 delta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 Total Chlordane ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 4,4'-DDD ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 -

Newsletter 3Rd Quarter 2020
3rd Quarter September 17,2020 PRESIDENT’S LETTER When I took art welding under Tom Gingras at ACC, it was a great as well as transformative experience. Not only did I learn to sculpt, but also found out I could sculpt. I had the best of teachers, but don’t tell Tom I said that, it will just go to his head. I, of course, was the greatest student he ever had. One of the most important things I learned from Tom was that when you start on a new sculpture, you will have drawn it out or otherwise framed it in your head, maybe downloaded a few pictures to guide you and you start--with enthusiasm--sometime by forcing yourself. After a while, a partial frame begins to take place and before you know it you are about 20-30% along. Then you hit a wall where you hate your work and want to tear it down or commit it to the back corner. However, Tom said, if you will just push on by this negative creative barrier, (there must be a psychological name for it) your work will “come together”. When finished, even if it turns out different than you imagined, it becomes acceptable or God forbid, you may even like it. I have found Tom’s words to be true for me time and again and I am grateful for his insight. Patience with oneself needs to be learned as well as patience with others. So in this age of Covid, we are trying to be patient. -

The Ovarian Cancer Research Fund Alliance SCOTUS
Nos. 14-1418, 14-1453, 14-1505, 15-35, 15-105, 15-119 & 15-191 IN THE Supreme Court of the United States _________ DAVID A. ZUBIK, ET AL., Petitioners, v. SYLVIA BURWELL, ET AL., Respondents. [CAPTIONS CONTINUED ON INSIDE COVER] _________ On Writs of Certiorari to the United States Courts of Appeals for the Third, Fifth, Tenth, and D.C. Circuits _________ APPENDIX VOL. II OF II OF AMICI CURIAE THE OVARIAN CANCER RESEARCH FUND ALLIANCE AND ITS PARTNER MEMBERS AND SCIENTIFIC ADVISORS’ BRIEF IN SUPPORT OF RESPONDENTS _________ JESSICA L. ELLSWORTH* Counsel of Record JESSICA B. LIVINGSTON MICHELLE A. KISLOFF HOGAN LOVELLS US LLP ANDREW S. FURLOW 1200 Seventeenth Street LOWELL M. ZETA Suite 1500 HOGAN LOVELLS US LLP Denver, CO 80202 555 Thirteenth Street, N.W. Washington, D.C. 20004 (202) 637-5886 [email protected] Counsel for Amici Curiae _________ PRIESTS FOR LIFE, ET AL., Petitioners, v. DEPARTMENT OF HEALTH & HUMAN SERVICES, ET AL., Respondents. _________ ROMAN CATHOLIC ARCHBISHOP OF WASHINGTON, ET AL., Petitioners, v. SYLVIA BURWELL, ET AL., Respondents. _________ EAST TEXAS BAPTIST UNIVERSITY, ET AL., Petition- ers, v. SYLVIA BURWELL, ET AL., Respondents. _________ LITTLE SISTERS OF THE POOR HOME FOR THE AGED, DENVER , COLORADO, ET AL., Petitioners, v. SYLVIA BURWELL, ET AL., Respondents. _________ SOUTHERN NAZARENE UNIVERSITY, ET AL., Petition- ers, v. SYLVIA BURWELL, ET AL., Respondents. _________ GENEVA COLLEGE, Petitioner, v. SYLVIA BURWELL, ET AL., Respondents. _________ 267a APPENDIX N ________ EUROPEAN JOURNAL OF CANCER 46 (2010) 2275-2284 ________ ORAL CONTRACEPTIVE USE AND BREAST OR OVARIAN CANCER RISK IN BRCA1/2 CARRIERS: A META-ANALYSIS ________ S.