Chlordane and Heptachlor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Serum Malondialdehyde Is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women
antioxidants Article Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women Shira Zelber-Sagi 1,2,*, Dana Ivancovsky-Wajcman 1, Naomi Fliss-Isakov 2,3, Michal Hahn 4, 2,3 2,3 2,3, 4, Muriel Webb , Oren Shibolet , Revital Kariv y and Oren Tirosh y 1 School of Public Health, University of Haifa, Haifa 3498838, Israel; [email protected] 2 Department of Gastroenterology, Tel Aviv Medical Center, Tel Aviv 6423914, Israel; naomifl@tlvmc.gov.il (N.F.-I.); [email protected] (M.W.); [email protected] (O.S.); [email protected] (R.K.) 3 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 4 Institute of Biochemistry, Food Science and Nutrition, The RH Smit Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rechovot 76100001, Israel; [email protected] (M.H.); [email protected] (O.T.) * Correspondence: [email protected]; Tel.: +972-3-6973984 The last two authors equally contributed to the paper. y Received: 25 May 2020; Accepted: 25 June 2020; Published: 2 July 2020 Abstract: Background: Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) are associated with increased oxidative stress and lipid peroxidation, but large studies are lacking. The aim was to test the association of malondialdehyde (MDA), as a marker of oxidative damage of lipids, with NAFLD and liver damage markers, and to test the association between dietary vitamins E and C intake and MDA levels. Methods: A cross-sectional study was carried out among subjects who underwent blood tests including FibroMax for non-invasive assessment of NASH and fibrosis. -

Indene CH Activation, Indole Π Vs. Nitrogen Lone-Pair Coordinati
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Organometallics Manuscript Author . Author Manuscript Author manuscript; available in PMC 2016 April 15. Published in final edited form as: Organometallics. 2007 ; 26(2): 281–287. doi:10.1021/om0606643. Reactions of Indene and Indoles with Platinum Methyl Cations: Indene C-H Activation, Indole π vs. Nitrogen Lone-Pair Coordination Travis J. Williams, Jay A. Labinger, and John E. Bercaw Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Pasadena, CA 91125 (U. S. A.) Abstract Reactions of indene and various substituted indoles with [(diimine)PtII(Me)(TFE)]+ cations have been studied (diimine = ArN = C(Me) − C(Me) = NAr; TFE = 2,2,2-trifluoroethanol). Indene displaces the TFE ligand from platinum to form a stable π coordination complex that, upon heating, undergoes C-H activation with first order kinetics, ΔH‡ = 29 kcal/mol, ΔS‡ = 10 eu, and a kinetic isotope effect of 1.1 at 60 °C. Indoles also initially form coordination complexes through the C2=C3 olefin, but these undergo rearrangement to the corresponding N-bound complexes. The relative rates of initial coordination and rearrangement are affected by excess acid or methyl substitution on indole. Introduction Selective C-H bond activation is a potentially valuable approach to synthetic problems in areas ranging from fuels and bulk chemicals to fine chemicals and pharmaceutical synthesis. 1 Studies of C-H activation in our laboratory have focused on models of the Shilov system,2 particularly [(diimine)PtII(Me)(solv)]+ (2, diimine = ArN = C(Me) − C(Me) = NAr; solv = 2,2,2-trifluoroethanol (TFE), H2O).3 These cations are capable of activating a variety of II carbon-hydrogen bonds.4 Cations 2 can be generated by protonolysis of (diimine)Pt Me2 species 1 in TFE with aqueous HBF43, 4ab or BX3 (X = C6F5,4cd F5), the latter producing H+ by boron coordination to TFE.4c In deuterated solvent 2 is formed as a mixture of two isotopologs. -

A COMPARATIVE STUDY of the CYTOGENETIC EFFECTS of the INSECTICIDES HEPTACHLOR, MALATHION, and METHYL PARATHION in Vicia Faba
Contam. Amb. 1, 7-16, 1985 A COMPARATIVE STUDY OF THE CYTOGENETIC EFFECTS OF THE INSECTICIDES HEPTACHLOR, MALATHION, AND METHYL PARATHION IN Vicia faba SANDRAGOMEZ-ARROYO, ANA MAR~ABAIZA, GRA~IELALOPEZ AND RAFAELVILLALOBOS- PIETRINI Laboratorio de Citogenética y Mutagénesis Am- bientales, Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Co- yoacán 04510, México, D. F. y Centro de Inves- tigación y Reproducción Animal, Universidad Au- tónoma de Tlaxcala. ABSTRACT In order to determine the potential cytogenetic effects of Heptachlor, Malathion and Methyl Parathion, meristems of broad bean root tips were treated with se- vera1 concentrations o£ these insecticides for different periods of treatment and recovery. Heptachlor and Malathion induced chromosomal alterations in anaphase cells as fragments and bridges, chromosomes with inactivated centromeres and isochroinosomes, and as micronuclei in interphase cells. Multipolar anaphases also appeared when the mitotic spindle was damaged. Heptachlor induced pycnosis but Malathion did not. Chromosomal aberrations caused by Methyl Parathion were observed in cells only during metaphase, since a strong c-mitotic effect was induced mostly in the form of fragments. Longer recovery periods (42 and 44 h) revealed tetraploid and pycnotic cells. Only in the case of Malation was there an increase in chromosome altera- tions with higher concentrations. However, no such dose response relationship was observed for micronuclei frequency. RESUMEN Con el fin de establecer el daño genético que ocasionan el Heptacloro, el Mala- tión y el Metil Paratión, se realizaron tratamientos sobre las células meristemá- ticas de la raíz de haba con diversas concentraciones de estos insecticidas y dife- rentes tiempos de tratamiento y recuperación. -

TOXICOLOGICAL PROFILE for HEPTACHLOR and HEPTACHLOR EPOXIDE
TOXICOLOGICAL PROFILE FOR HEPTACHLOR and HEPTACHLOR EPOXIDE Prepared by: Syracuse Research Corporation Under Contract No. 200-2004-09793 Prepared for: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry November 2007 HEPTACHLOR AND HEPTACHLOR EPOXIDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HEPTACHLOR AND HEPTACHLOR EPOXIDE iii UPDATE STATEMENT A Toxicological Profile for Heptachlor/Heptachlor Epoxide, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 HEPTACHLOR AND HEPTACHLOR EPOXIDE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. -

Oxisresearch™ a Division of OXIS Health Products, Inc
OxisResearch™ A Division of OXIS Health Products, Inc. BIOXYTECHÒ MDA-586™ Spectrophotometric Assay for Malondialdehyde For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number 21044 INTRODUCTION The Analyte Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals, and is used as an indicator of oxidative stress in cells and tissues. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds. These include reactive carbonyl compounds, of which the most abundant is malondialdehyde (MDA). Therefore, measurement of malondialdehyde is widely used as an indicator of lipid peroxidation (1). Increased levels of lipid peroxidation products have been associated with a variety of chronic diseases in both humans (2, 3) and model systems (4, 5). MDA reacts readily with amino groups on proteins and other biomolecules to form a variety of adducts (1), including cross-linked products (6). MDA also forms adducts with DNA bases that are mutagenic (7, 8) and possibly carcinogenic (9). DNA-protein cross-links are another result of the reaction between DNA and MDA (10). The TBARS method is commonly used to measure MDA in biological samples (11). However, this reaction is relatively nonspecific; both free and protein-bound MDA can react. The MDA-586 method is designed to assay free MDA or, after a hydrolysis step, total MDA (i.e., free and protein-bound Schiff base conjugates). The assay conditions serve to minimize interference from other lipid peroxidation products, such as 4- hydroxyalkenals. PRINCIPLES OF THE PROCEDURE The MDA-586 method1 (12) is based on the reaction of a chromogenic reagent, N-methyl-2- phenylindole (R1, NMPI), with MDA at 45°C. -

Neuroprotective Effects of Geniposide from Alzheimer's Disease Pathology
Neuroprotective effects of geniposide from Alzheimer’s disease pathology WeiZhen Liu1, Guanglai Li2, Christian Hölscher2,3, Lin Li1 1. Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China 2. Second hospital, Shanxi medical University, Taiyuan, PR China 3. Neuroscience research group, Faculty of Health and Medicine, Lancaster University, Lancaster LA1 4YQ, UK running title: Neuroprotective effects of geniposide corresponding author: Prof. Lin Li Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China Email: [email protected] Neuroprotective effects of geniposide Abstract A growing body of evidence have linked two of the most common aged-related diseases, type 2 diabetes mellitus (T2DM) and Alzheimer disease (AD). It has led to the notion that drugs developed for the treatment of T2DM may be beneficial in modifying the pathophysiology of AD. As a receptor agonist of glucagon- like peptide (GLP-1R) which is a newer drug class to treat T2DM, Geniposide shows clear effects in inhibiting pathological processes underlying AD, such as and promoting neurite outgrowth. In the present article, we review possible molecular mechanisms of geniposide to protect the brain from pathologic damages underlying AD: reducing amyloid plaques, inhibiting tau phosphorylation, preventing memory impairment and loss of synapses, reducing oxidative stress and the chronic inflammatory response, and promoting neurite outgrowth via the GLP-1R signaling pathway. In summary, the Chinese herb geniposide shows great promise as a novel treatment for AD. Key words: Alzheimer’s disease, geniposide, amyloid-β, neurofibrillary tangles, oxidative stress, inflammatation, type 2 diabetes mellitus, glucagon like peptide receptor, neuroprotection, tau protein Neuroprotective effects of geniposide 1. -

Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2
Technical Publication 91-01 Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2 by Richard J. Pfeuffer January 1991 This publication was produced at an annual cost of $243.75 or $.49 per copy to inform the public. 500 191 Produced on recycled paper. Water Quality Division Research and Evaluation Department South Florida Water Management District West Palm Beach, Florida A IBSTRAC'I' Pesticide monitoring data are collected under the requirements of several permits and agreements as an indicator of water quality. The monitoring provides data to determine shifts or adverse trends in the quality of water being delivered to Lake Okeechobee, Everglades National Park, and the Water Conservation Areas. In addition, pesticide residue data are collected throughout the South Florida Water Management District at locations selected to determine water quality conditions at the major water control points. Special investigations are performed on selected pesticides as required and follow-up sampling is conducted based on the pesticides detected. Data were collected from 13 stations in 1984. By 1988, the network was expanded to 29 stations. Currently, water and sediment samples are collected quarterly and analyzed for 67 pesticides, herbicides and degradation products. Out of a total of 197 surface water samples, 13 percent had detectable residues, while 25 percent of the 208 sediment samples had detectable residues. The compounds detected in the water samples included atrazine and zinc phosphide while a variety of compounds, including DDT, have been detected in the sediment. None of the residues detected are considered to have adverse health or environmental effects. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -

P-Listed Hazardous Wastes
P-Listed Hazardous Wastes The Environmental Protection Agency (EPA) has identified a number of chemicals on the EPA “P-list” that present an especially acute hazard when disposed of as hazardous waste. Because of their acute hazards, there are more stringent requirements when disposing of these wastes: ►Container size: When collecting p-listed chemicals as waste, the volume of the hazardous waste container must not exceed one quart (approximately one liter). ►Empty containers: Empty containers that held p-listed chemicals must also be disposed of as hazardous waste. They are not allowed to be washed or re-used. ►Contaminated materials: Disposable materials that become contaminated with p-listed chemicals (e.g. gloves, weighing boats, etc.) must also be disposed of as hazardous waste. Non-disposable materials must be “triple-rinsed”, or rinsed three times to remove the contamination. This rinsate must be collected as hazardous waste. Materials contaminated with p-listed chemicals may not be washed or re-used until they have been triple-rinsed. Remember: - Label the waste as hazardous waste. Most common p-listed wastes Just like all other hazardous wastes, p-listed Chemical CAS number wastes must be labeled with the words Acrolein 107–02–8 “hazardous waste”, the complete chemical Allyl alcohol 107–18–6 name, and the associated hazard Arsenic compounds Varies characteristics (e.g., ignitable, corrosive, Inorganic cyanide Varies toxic, or reactive). salts Carbon disulfide 75-15-0 - Use disposable materials whenever Cyanogen and 460-19-5, 506-77-4 possible. Triple-rising non-disposable Cyanogen Chloride material generates a lot of waste, which can 2,4-Dinitrophenol 51–28–5 be difficult to dispose of safely. -

Phenethylamine in Chlorella Alleviates High-Fat Diet-Induced Mouse Liver
www.nature.com/npjscifood ARTICLE OPEN Phenethylamine in chlorella alleviates high-fat diet-induced mouse liver damage by regulating generation of methylglyoxal ✉ Yifeng Zheng1, Agustin Martin-Morales1, Jing Wang1, Masaki Fujishima 2, Eri Okumura 2 and Kenji Sato 1 This study examined the effects of oral administration of water extract of chlorella (WEC) (100 mg/kg bodyweight) and phenethylamine (10 μg/kg bodyweight) on high-fat diet (HFD)-induced liver damage in mice. Phenethylamine significantly mitigated HFD-induced lipid oxidation (generation of malondialdehyde) and liver damage without markedly decreasing hepatic lipid accumulation. WEC exerted similar effects although with decreased efficacy. In addition, WEC and phenethylamine decreased the methylglyoxal levels and increased the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels in the liver. Methylglyoxal is generated from substrates of GAPDH, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. These facts indicate that methylglyoxal triggers oxidation of accumulated lipid, which generates malondialdehyde and consequently induces liver damage. Suppression of generation of toxic aldehydes by WEC and phenethylamine was also confirmed by maintaining hepatic cysteine, highly reactive to aldehydes. Thus, trace amounts of phenethylamine alleviate HFD-induced liver damage by regulating methylglyoxal via increase of GAPDH. npj Science of Food (2021) 5:22 ; https://doi.org/10.1038/s41538-021-00105-3 1234567890():,; INTRODUCTION lipid deposition-induced oxidative stress in the liver contributes to Chlorella pyrenoidosa, a freshwater unicellular green alga, and its the pathogenesis of NAFLD13,14, whereas the liver contains potent water extract have a long history of usage as food supplements. antioxidant enzyme systems, such as the superoxide dismutase Various animal studies and clinical trials have reported that C. -
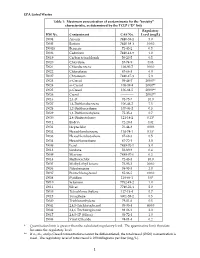
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Protective Effects of Geniposide and Genipin Against Hepatic Ischemia/Reperfusion Injury in Mice
Original Article Biomol Ther 21(2), 132-137 (2013) Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice Joonki Kim1, Hyo-Yeon Kim2 and Sun-Mee Lee2,* 1Natural Medicine Center, Korea Institute of Science & Technology, Gangneung 210-340, 2School of Pharmacy, Sungkyunkwan University, Suwon 440-746, Republic of Korea Abstract Geniposide is an active product extracted from the gardenia fruit, and is one of the most widely used herbal preparations for liver disorders. This study examined the cytoprotective properties of geniposide and its metabolite, genipin, against hepatic ischemia/ reperfusion (I/R) injury. C57BL/6 mice were subjected to 60 min of ischemia followed by 6 h of reperfusion. Geniposide (100 mg/ kg) and genipin (50 mg/kg) were administered orally 30 min before ischemia. In the I/R mice, the levels of serum alanine amino- transferase and hepatic lipid peroxidation were elevated, whereas hepatic glutathione/glutathione disulfide ratio was decreased. These changes were attenuated by geniposide and genipin administration. On the other hand, increased hepatic heme oxygen- ase-1 protein expression was potentiated by geniposide and genipin administration. The increased levels of tBid, cytochrome c protein expression and caspase-3 activity were attenuated by geniposide and genipin. Increased apoptotic cells in the I/R mice were also significantly reduced by geniposide and genipin treatment. Our results suggest that geniposide and genipin offer signifi- cant hepatoprotection against I/R injury by reducing oxidative stress and apoptosis. Key Words: Geniposide, Genipin, Ischemia, Reperfusion, Liver, Apoptosis INTRODUCTION 2004). Oral administration of geniposide increased hepatic glutathione (GSH) content, which is responsible for hepatopro- Ischemia/reperfusion (I/R) injury to the liver is of clinical im- tection against aflatoxin B1-induced liver injury in rats (Kanget portance in humans after hemorrhagic and cardiogenic shock, al., 1997).