Download in English
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multi-Scale Impact of Chronic Exposure to Environmental Concentrations Of
Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta Romain Colpaert, Pierre-Henri Villard, Laetitia de Jong, Marina Mambert, Karim Benbrahim, Joelle Abraldes, Claire Cerini, Valérie Pique, Maxime Robin, Xavier Moreau To cite this version: Romain Colpaert, Pierre-Henri Villard, Laetitia de Jong, Marina Mambert, Karim Benbrahim, et al.. Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta. Environmental Science and Pollution Research, Springer Verlag, 2020, 27 (33), pp.41052-41062. 10.1007/s11356-019-06859-4. hal-02451113 HAL Id: hal-02451113 https://hal-amu.archives-ouvertes.fr/hal-02451113 Submitted on 23 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta. Romain COLPAERT1, Pierre-Henri VILLARD1, Laetitia DE JONG1, Marina MAMBERT1, Karim BENBRAHIM1, Joelle ABRALDES1, Claire CERINI2, Valérie PIQUE1, Maxime ROBIN1, Xavier MOREAU1 1 : Aix Marseille Univ, Avignon Univ, CNRS, IRD, IMBE, Marseille, France 2 : Aix Marseille Univ, Inserm U1263, C2VN, Marseille, France Corresponding author: email : [email protected] phone : +33-(0)4-91-83-56-38 Abstract Chlordecone (CLD) is an organochlorine pesticide widely used by the past to control pest insects in banana plantations in the French West Indies. -

A COMPARATIVE STUDY of the CYTOGENETIC EFFECTS of the INSECTICIDES HEPTACHLOR, MALATHION, and METHYL PARATHION in Vicia Faba
Contam. Amb. 1, 7-16, 1985 A COMPARATIVE STUDY OF THE CYTOGENETIC EFFECTS OF THE INSECTICIDES HEPTACHLOR, MALATHION, AND METHYL PARATHION IN Vicia faba SANDRAGOMEZ-ARROYO, ANA MAR~ABAIZA, GRA~IELALOPEZ AND RAFAELVILLALOBOS- PIETRINI Laboratorio de Citogenética y Mutagénesis Am- bientales, Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Co- yoacán 04510, México, D. F. y Centro de Inves- tigación y Reproducción Animal, Universidad Au- tónoma de Tlaxcala. ABSTRACT In order to determine the potential cytogenetic effects of Heptachlor, Malathion and Methyl Parathion, meristems of broad bean root tips were treated with se- vera1 concentrations o£ these insecticides for different periods of treatment and recovery. Heptachlor and Malathion induced chromosomal alterations in anaphase cells as fragments and bridges, chromosomes with inactivated centromeres and isochroinosomes, and as micronuclei in interphase cells. Multipolar anaphases also appeared when the mitotic spindle was damaged. Heptachlor induced pycnosis but Malathion did not. Chromosomal aberrations caused by Methyl Parathion were observed in cells only during metaphase, since a strong c-mitotic effect was induced mostly in the form of fragments. Longer recovery periods (42 and 44 h) revealed tetraploid and pycnotic cells. Only in the case of Malation was there an increase in chromosome altera- tions with higher concentrations. However, no such dose response relationship was observed for micronuclei frequency. RESUMEN Con el fin de establecer el daño genético que ocasionan el Heptacloro, el Mala- tión y el Metil Paratión, se realizaron tratamientos sobre las células meristemá- ticas de la raíz de haba con diversas concentraciones de estos insecticidas y dife- rentes tiempos de tratamiento y recuperación. -

OECD Environment Health and Safety Publications Series on Testing and Assessment No
OECD Environment Health and Safety Publications Series on Testing and Assessment No. 21 Detailed Review Paper Appraisal of Test Methods for Sex Hormone Disrupting Chemicals Environment Directorate ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT Paris May 2001 1 Also Published in the Series Testing and Assessment: No. 1, Guidance Document for the Development of OECD Guidelines for Testing of Chemicals (1993; reformatted 1995) No. 2, Detailed Review Paper on Biodegradability Testing (1995) No. 3, Guidance Document for Aquatic Effects Assessment (1995) No. 4, Report of the OECD Workshop on Environmental Hazard/Risk Assessment (1995) No. 5, Report of the SETAC/OECD Workshop on Avian Toxicity Testing (1996) No. 6, Report of the Final Ring-test of the Daphnia magna Reproduction Test (1997) No. 7, Guidance Document on Direct Phototransformation of Chemicals in Water (1997) No. 8, Report of the OECD Workshop on Sharing Information about New Industrial Chemicals Assessment (1997) No. 9 Guidance Document for the Conduct of Studies of Occupational Exposure to Pesticides During Agricultural Application (1997) No. 10, Report of the OECD Workshop on Statistical Analysis of Aquatic Toxicity Data (1998) No. 11, Detailed Review Paper on Aquatic Testing Methods for Pesticides and industrial Chemicals (1998) No. 12, Detailed Review Document on Classification Systems for Germ Cell Mutagenicity in OECD Member Countries (1998) No. 13, Detailed Review Document on Classification Systems for Sensitising Substances in OECD Member Countries 1998) No. 14, Detailed Review Document on Classification Systems for Eye Irritation/Corrosion in OECD Member Countries (1998) No. 15, Detailed Review Document on Classification Systems for Reproductive Toxicity in OECD Member Countries (1998) No. -

TOXICOLOGICAL PROFILE for HEPTACHLOR and HEPTACHLOR EPOXIDE
TOXICOLOGICAL PROFILE FOR HEPTACHLOR and HEPTACHLOR EPOXIDE Prepared by: Syracuse Research Corporation Under Contract No. 200-2004-09793 Prepared for: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry November 2007 HEPTACHLOR AND HEPTACHLOR EPOXIDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HEPTACHLOR AND HEPTACHLOR EPOXIDE iii UPDATE STATEMENT A Toxicological Profile for Heptachlor/Heptachlor Epoxide, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 HEPTACHLOR AND HEPTACHLOR EPOXIDE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. -

(A) Sources, Including As Appropriate (Provide Summary Information
UNEP/POPS/POPRC.1/4 Format for submitting pursuant to Article 8 of the Stockholm Convention the information specified in Annex E of the Convention Introductory information Name of the submitting Party/observer NGO Observer: Pesticide Action Network on behalf of the International POPs Elimination Network (IPEN) Contact details Clare Butler Ellis PhD, M.Inst.P, C.Env. Pesticide Action Network UK [email protected] Joseph DiGangi, PhD Environmental Health Fund +001-312-566-0985 [email protected] Chemical name Chlordecone Chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-octahydro-1,3,4-metheno-2H- cyclobuta[cd]pentalen-2-one CAS=143-50-0 Common trade names: GC 1189, Kepone, Merex Synonyms: Chlordecone, Chlordecone Kepone, Decachloroketone, Decachlorooctahydro-1,3,4-metheno-2H-cyclobuta(cd)pentalen-2-one, Decachloropentacyclo(5.3.0.0.0.0 2,6,4,10,5,9)decane-3-one, Decachlorotetracyclodecanone decachlorooctahydro- , Date of submission 27 January 2006 (a) Sources, including as appropriate (provide summary information and relevant references) (i) Production data: Quantity 1 “Chlordecone is no longer produced commercially in the United States. Between 1951 and 1975, approximately 3.6 million pounds (1.6 million kg) of chlordecone were produced in the United States (Epstein 1978). During this period, Allied Chemical Annex E information on chlordecone 1 UNEP/POPS/POPRC.1/4 Company produced approximately 1.8 million pounds (816,500 kg) of chlordecone at plants in Claymont, Delaware; Marcus Hook, Pennsylvania and Hopewell, Virginia. In 1974, because of increasing demand for chlordecone and a need to use their facility in Hopewell, Virginia, for other purposes, Allied Chemical transferred its chlordecone manufacturing to Life Sciences Products Company (EPA 1978b). -

Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2
Technical Publication 91-01 Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2 by Richard J. Pfeuffer January 1991 This publication was produced at an annual cost of $243.75 or $.49 per copy to inform the public. 500 191 Produced on recycled paper. Water Quality Division Research and Evaluation Department South Florida Water Management District West Palm Beach, Florida A IBSTRAC'I' Pesticide monitoring data are collected under the requirements of several permits and agreements as an indicator of water quality. The monitoring provides data to determine shifts or adverse trends in the quality of water being delivered to Lake Okeechobee, Everglades National Park, and the Water Conservation Areas. In addition, pesticide residue data are collected throughout the South Florida Water Management District at locations selected to determine water quality conditions at the major water control points. Special investigations are performed on selected pesticides as required and follow-up sampling is conducted based on the pesticides detected. Data were collected from 13 stations in 1984. By 1988, the network was expanded to 29 stations. Currently, water and sediment samples are collected quarterly and analyzed for 67 pesticides, herbicides and degradation products. Out of a total of 197 surface water samples, 13 percent had detectable residues, while 25 percent of the 208 sediment samples had detectable residues. The compounds detected in the water samples included atrazine and zinc phosphide while a variety of compounds, including DDT, have been detected in the sediment. None of the residues detected are considered to have adverse health or environmental effects. -

Research Article Effect of Chlordecone on The
NorCal Open Access Publications Journal of Aquatic Research and NORCAL Marine Sciences OPEN ACCESS PUBLICATION Volume 2; Issue 2 Asifa KP and Chitra KC Research Article ISSN 2639-4618 Effect of Chlordecone on the Reproductive Potential of the Cichlid Fish, Pseudetroplus Maculatus (Bloch, 1795) Asifa KP, Chitra KC* Endocrinology and Toxicology Laboratory, Department of Zoology, University of Calicut, Kerala, India. *Corresponding author: Chitra KC, Endocrinology and Toxicology Laboratory, Department of Zoology, University of Calicut, Kerala, India. Email: [email protected] Received Date: 16 March, 2019; Accepted Date: 03 May, 2019; Published Date: 17 May, 2019 Abstract Introduction The present study was undertaken to evaluate that chlordecone, During the past few decades, large number of environmental an organochlorine pesticide, possessing estrogenic properties contaminants such as, pesticides, pharmaceutical drugs, plas- are known to influence the reproductive potential of the cich- tic products, natural phytochemicals etc. has been continu- lid fish, Pseudetroplus maculatus. Chlordecone at two sublethal ously released into various compartments of both terrestrial concentrations, 3.5 and 7µg/L, were exposed to fish for 4, 7, 15 and aquatic ecosystems, which are known to disrupt the en- and 30 days in order to evaluate the level of vitellogenin, main- docrine system of animals, including humans. Such chemicals taining the control groups. Vitellogenin concentrations were are called endocrine-disrupting chemicals (EDCs), which can measured in both male and female fishes by using indirect end- interfere with biosynthesis, transport, action, metabolism and points, such as alkali-labile phosphoprotein (ALP), total pro- removal of various hormones in the body resulting in varia- tein and calcium concentrations in the blood plasma. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -

P-Listed Hazardous Wastes
P-Listed Hazardous Wastes The Environmental Protection Agency (EPA) has identified a number of chemicals on the EPA “P-list” that present an especially acute hazard when disposed of as hazardous waste. Because of their acute hazards, there are more stringent requirements when disposing of these wastes: ►Container size: When collecting p-listed chemicals as waste, the volume of the hazardous waste container must not exceed one quart (approximately one liter). ►Empty containers: Empty containers that held p-listed chemicals must also be disposed of as hazardous waste. They are not allowed to be washed or re-used. ►Contaminated materials: Disposable materials that become contaminated with p-listed chemicals (e.g. gloves, weighing boats, etc.) must also be disposed of as hazardous waste. Non-disposable materials must be “triple-rinsed”, or rinsed three times to remove the contamination. This rinsate must be collected as hazardous waste. Materials contaminated with p-listed chemicals may not be washed or re-used until they have been triple-rinsed. Remember: - Label the waste as hazardous waste. Most common p-listed wastes Just like all other hazardous wastes, p-listed Chemical CAS number wastes must be labeled with the words Acrolein 107–02–8 “hazardous waste”, the complete chemical Allyl alcohol 107–18–6 name, and the associated hazard Arsenic compounds Varies characteristics (e.g., ignitable, corrosive, Inorganic cyanide Varies toxic, or reactive). salts Carbon disulfide 75-15-0 - Use disposable materials whenever Cyanogen and 460-19-5, 506-77-4 possible. Triple-rising non-disposable Cyanogen Chloride material generates a lot of waste, which can 2,4-Dinitrophenol 51–28–5 be difficult to dispose of safely. -

In Silico Pharmacodynamics, Toxicity Profile and Biological Activities of the Saharan Medicinal Plant Limoniastrum Feei
Brazilian Journal of Pharmaceutical Sciences Article http://dx.doi.org/10.1590/s2175-97902017000300061 In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei Ouahab Ammar* Department of Pharmacy, Faculty of Medical Sciences, University of Batna 2, Algeria In-silico study was performed to find the pharmacodynamics, toxicity profiles and biological activities of three phytochemicals isolated from Limoniastrum feei (Plumbagenaceae). Online pharmacokinetic tools were used to estimate the potential of Quercetin, kaempferol-3-O-β-D-glucopyranoside (astragalin) and quercitin-7-O-β-D-glucopyranoside as specific drugs. Then the prediction of potential targets of these compounds were investigated using PharmMapper. Auto-Dock 4.0 software was used to investigate the different interactions of these compounds with the targets predicted earlier. The permeability of quercetin s rule of five. Hematopoietic prostaglandin (PG) D׳ was found within the range stated by Lipinski synthase (HPGDS), farnesyl diphosphate synthetase (FPPS) and the deoxycytidine kinase (DCK) were potential targets for quercetin, astragalin and quercetin 7, respectively. Quercetin showed antiallergic and anti-inflammatory activity, while astragalin and quercetin 7 were predicted to have anticancer activities. The activity of Astragalin appeared to be mediated by FPPS inhibition. The inhibition of DCK was predicted as the anticancer mechanisms of quercetin 7. The compounds showed interesting interactions and satisfactory binding energies when docked into their targets. These compounds are proposed to have activities against a variety of human aliments such as allergy, tumors, muscular dystrophy, and diabetic cataracts. Keywords: Limoniastrum feei/pharmacokinetics. Limoniastrum feei/biological activity. Quercetin. Astragalin. Quercetin 7. Medicinal plants. Molecular docking. -
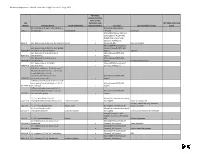
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10-