Chlordane and Toxaphene Residues Following Cooking of Treated Channel Catfish Fillets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -
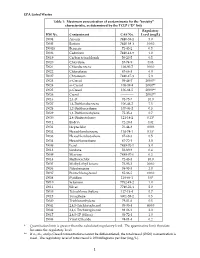
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Health Consultation Chevron Chemical Co
- • ! t Health Consultation Chevron Chemical Co. (Ortho Division) Orlando, Orange County, Florida CERCLIS NO. FLD004064242 June 1995 Prepared by Environmental Toxicology The Florida Department of Health and Rehabilitative Services Under a Cooperative Agreement With Agency for Toxic Substances and Disease Registry U.S. Public Health Service Department of Health and Human Services - ' ' Health Consultation - Chevron Chemical June 1995 Background and Statement of Issues The purpose of this health consultation is to interpret the results of indoor air monitoring for pesticides in two trailers adjacent to the Chevron Chemical Superfund site in Orlando, Florida. During a March 9, 1995 public meeting, two nearby residents expressed concerns that the insides of their trailers were contaminated with pesticides. We agreed to test their trailers for pesticide contamination. The Chevron Chemical Co. (Ortho Division) Superfund site is a former pesticide formulation plant and truck repair facility in Orlando, Florida (Figures 1-3, Appendix A). Past waste disposal practices contaminated soil and ground water. Stormwater run-off carried pesticide-contaminated soil to the adjacent Armstrong Trailer Park. In 1992, the Chevron Chemical Company removed the on-site contaminated soil. In 1994, they removed the contaminated soil from the Armstrong Trailer Park. In a 1995 public health assessment (ATSDR 1995), we found the site was a public health hazard because some residents of the adjacent Armstrong Trailer Park may have unknowingly eaten small amounts of soil contaminated with the pesticide chlordane. As a result, we estimated those residents have a moderately increased risk of liver cancer. Since Chevron cleaned up the chlordane-contaminated soil at this trailer park, we estimated the remaining cancer risk from chlordane is insignificant. -

Chronology of Pesticides Used on National Park Service Collections
Conserve O Gram June 2001 Number 2/16 Chronology Of Pesticides Used On National Park Service Collections The history of National Park Service pesticide use publication). Synonyms and trade names were policy for museum collection objects is obtained from the Merck Index, notes from the documented in various publications including IPM Coordinator, and two Internet sites Field Manual for Museums (Burns), Manual for (<http://chemfinder.com> and <http:// Museums (Lewis), versions of the Museum www.cdpr.ca.gov/cgi-bin/epa>). Handbook, Part I, and two versions of the Integrated Pest Management Information Manual. Not all of the chemicals listed in the Other non-policy sources include Coleman's accompanying charts were marketed as pesticides. Manual for Small Museums, object treatment Some are fungicides and microbiocides. One, reports and notes from NPS staff, and notes from Lexol, is a leather preservative and consolidant. the Office of the Integrated Pest Management All of these are included here because records (IPM) Coordinator. indicate they were applied to objects as pesticides. The two accompanying charts list the types of The potential for pesticide residue remaining on pesticides that may have been used on National collection objects is very high. Objects with such Park Service collections along with some common residues pose a health risk to curatorial staff and synonyms and trade names. to the public who come into physical contact with them, unless proper precautions are taken. Dates shown in blue on the chart represent Additional information on health and safety issues published recommendations for the use of and protective measures can be found in the pesticides. -

TOXICOLOGICAL PROFILE for CHLORDANE U.S. DEPARTMENT of HEALTH and HUMAN SERVICES Public Health Service Agency for Toxic Subs
TOXICOLOGICAL PROFILE FOR CHLORDANE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 1994 CHLORDANE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. CHLORDANE iii UPDATE STATEMENT A Toxicological Profile for Chlordane was released on December 1989. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 CHLORDANE vii CONTRIBUTORS CHEMICAL MANAGERS(S)/AUTHOR(S): Henry G. Abadin, M.S.P.H. ATSDR, Division of Toxicology, Atlanta, GA Ronald Baynes, D.V.M., M.S. Paul F. Goetchius, D.V.M. Syracuse Research Corporation, Syracuse, NY THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying endpoints. 3 . Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. 4 . Quality Assurance Review. The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problems in format or content, and establishes that Guidance has been followed. -

Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery
COMMON HOUSEHOLD HAZARDOUS WASTES CORROSIVES (ACIDS) Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery Acid Household Waxes Arsinic Compounds Copper Cleaners Isopropyl Alcohol Automotive Cleaners Etching Solutions Kerosene Bacterial Pipe Cleaners Ferric Chloride Lacquer Thiner Bordeaux Mix Hydrochloric Acid Lacquer Paint (unsolidified) Boric Acid Hydrofluoric Acid Linseed Oil Bug Removers Metal Cleaners Liquid Waxes Chlordane Muriatic Acid Liquid Sandpaper Chromium Navel Jelly Liquid Butane Copper Sulfate Phosphoric Acid Methanol DDT Pool Acid Naphtha Diazinon Sodium Bisulfate Oils (petroleum) Dimethylamine Salts Sulfuric Acid Organic Solvents Disinfectants Toilet Bowl Cleaners Paint Thinners Dog Repellent Paint Strippers Fertilizers CORROSIVES (BASES) Paraffin Oil Flea Spray/Powders Ammonia Perfume Fluorine Ammonia Based Cleaners Petroleum Distillers Fungicides Battery Terminal Cleaners Plastic Roof Cement Gopher Killer Caustic Soda Plastic Model Cement Hydrofluoric Acid Cess Pool Cleaners Polyurethane Paint (unsolidified) Insect Sprays Drain Cleaners Polyurethane Cement (unsolid.) Lead Compounds Household Cleaners Powe Steering Fluid Lice Powders Lime Primers Lindane Lye Roofing Cement Malathion Oven Cleaners Rug/Upholstery Cleaner Methylene Chloride Sodium Hydroxide Sealers Mole Killer Window Cleaners Shellac Thinner Moth Crystals Silicone Sprays Pesticides FLAMMABLES & COMBUSTIBLES Spot Remover/Dry Clean Fluids Pharmaceuticals Acetone Thinner Plant Food Adhesives Tile Cement Pruning Paint Aerosol Tire Black Pyethrins -

Table 1 Summary of Soil Analytical Methods
TABLE 1 SUMMARY OF SOIL ANALYTICAL METHODS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California No. of Analyzed Compound EPA Method Soil Samples Organochlorine Pesticides (OCPs) EPA Method 8081A 26 Total Lead EPA Method 6010B 30 Arsenic EPA Method 6010B 73 Page 1 of 1 TABLE 2 SUMMARY OF SOIL ANALYTICAL RESULTS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California Number. of Number of Analyzed Range of Analyzed Samples with Compounds Detections Samples Detections Organochlorine Pesticides 26 2 2-26 ug/kg a-chlordane 26 2 both 5 ug/kg d-chlordane 26 2 3-4 ug/kg total chlordane 26 2 11-26 ug/kg dieldrin 26 1 2 ug/kg Total Lead 30 29 1-53 mg/kg Arsenic 73 46 1-34 mg/kg mg/kg - milligrams per kilogram ug/kg - micrograms per kilogram mdl - method detection limit Page 1 of 1 TABLE 3 SUMMARY OF SOIL ANALYTICAL RESULTS PESTICIDES CRENSHAW HIGH SCHOOL 5010 11the Avenue Los Angeles, California Compound PB-2-6" PB-3-6" PB-5-6" PB-8-6" PB-12-6" PB-13-6" PB-18-6" EPA Method 8081A (ug/kg) Aldrin ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-BHC (Lindane) ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 beta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 delta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 Total Chlordane ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 4,4'-DDD ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 -

Chlordane in Drinking-Water
WHO/SDE/WSH/03.04/84 English only Chlordane in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality © World Health Organization 2004 Requests for permission to reproduce or translate WHO publications - whether for sale of for non- commercial distribution - should be addressed to Publications (Fax: +41 22 791 4806; e-mail: [email protected]. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damage incurred as a results of its use. Preface One of the primary goals of WHO and its member states is that “all people, whatever their stage of development and their social and economic conditions, have the right to have access to an adequate supply of safe drinking water.” A major WHO function to achieve such goals is the responsibility “to propose ... regulations, and to make recommendations with respect to international health matters ....” The first WHO document dealing specifically with public drinking-water quality was published in 1958 as International Standards for Drinking-water. -

Toxicological Review of Chlordane (Technical)
TOXICOLOGICAL REVIEW of CHLORDANE (TECHNICAL) (CAS No. 12789-03-6) In Support of Summary Information on the Integrated Risk Information System (IRIS) December 1997 U.S. Environmental Protection Agency Washington, DC TABLE OF CONTENTS Authors and Reviewers ...................................................... iv Foreword ................................................................ vii 1.0 Introduction ...........................................................1 2.0 Toxicokinetics Relevant to Assessments .....................................2 3.0 Absorption, Metabolism, Distribution, Excretion, and Toxicokinetics .............3 4.0 Hazard Identification ....................................................5 4.1 Studies in Humans ....................................................5 4.1.1 Noncancer Effects in Humans .......................................5 4.1.2 Cancer Effects in Humans .........................................9 4.1.2.1 Case-control studies ........................................9 4.1.2.2 Occupational cohort studies .................................11 4.1.2.3 Case reports .............................................13 4.2 Subchronic and Chronic Studies and Cancer Bioassays in Animals—Oral and Inhalation ......................................................15 4.3 Reproductive/Developmental Studies—Oral and Inhalation .................... 26 4.4 Other Toxicologically Relevant Studies ...................................30 4.4.1 Mechanistic Studies .............................................30 4.4.2 Genotoxicity ..................................................30 -
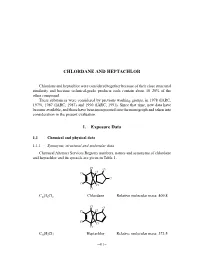
Chlordane and Heptachlor
CHLORDANE AND HEPTACHLOR Chlordane and heptachlor were considered together because of their close structural similarity and because technical-grade products each contain about 10–20% of the other compound. These substances were considered by previous working groups, in 1978 (IARC, 1979), 1987 (IARC, 1987) and 1990 (IARC, 1991). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Chemical Abstract Services Registry numbers, names and synonyms of chlordane and heptachlor and its epoxide are given in Table 1. Cl Cl Cl Cl Cl Cl Cl Cl C10H6Cl8 Chlordane Relative molecular mass: 409.8 Cl Cl Cl Cl Cl Cl Cl C10H5Cl7 Heptachlor Relative molecular mass: 373.5 –411– 412 IARC MONOGRAPHS VOLUME 79 Table 1. Chemical Abstract Services Registry numbers, names and synonyms of chlordane, heptachlor and its epoxide Name CAS Reg. Nosa Chem. Abstr. namesb and synonyms Chlordane 57-74-9 ENT 9932; 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a- (39400-80-1); hexahydro-4,7-methano-1H-indene; 1,2,4,5,6,7,8,8- 53637-13-1) octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano- indene (IUPAC); octachloro-4,7-methanotetrahydro- indane; 1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetra- hydro-4,7-methanoindan; OMS 1437 Technical-grade 12789-03-6 chlordane cis-Chlordane 5103-71-9 α-Chlordan; α-chlordane; cis-chlordan; (152322-29-7; (1α,2α,3aα,4β,7β,7aα)-1,2,4,5,6,7,8,8-octachloro- 22212-52-8; -

EPA's Hazardous Waste Listing
Hazardous Waste Listings A User-Friendly Reference Document September 2012 Table of Contents Introduction ..................................................................................................................................... 3 Overview of the Hazardous Waste Identification Process .............................................................. 5 Lists of Hazardous Wastes .............................................................................................................. 5 Summary Chart ............................................................................................................................... 8 General Hazardous Waste Listing Resources ................................................................................. 9 § 261.11 Criteria for listing hazardous waste. .............................................................................. 11 Subpart D-List of Hazardous Wastes ............................................................................................ 12 § 261.31 Hazardous wastes from non-specific sources. ............................................................... 13 Spent solvent wastes (F001 – F005) ......................................................................................... 13 Wastes from electroplating and other metal finishing operations (F006 - F012, and F019) ... 18 Dioxin bearing wastes (F020 - F023, and F026 – F028) .......................................................... 22 Wastes from production of certain chlorinated aliphatic hydrocarbons (F024 -

Insect-Proofing During Building Construction
INSECT-PROOFING DURING BUILDING CONSTRUCTION W. EBELING * R. E. WAGNER - D. A. REIERSON TRUCTURAL AND HOUSEHOLD PEST mental evidence of efficiency of soil treat- foundation walls or piers. Specific in- S CONTROL in the United States re- ment have been the investigations of the structions for treatment for slab-on- quires annual expenditures of hundreds Southern Forest Experiment Station of ground, raised-foundation, and basement of millions of dollars and the services of the Forest Service, U. S. Department of construction may be obtained in publica- 27,000 licensed pest control operators. Agriculture. These investigations showed tions of the federal or state agricultural Preventive measures against structural that currently recommended dosages of experiment stations or among the tech- and household pests should be taken dur- certain chlorinated hydrocarbons formed nical releases of the National Pest Con- ing the construction of a building, be- a compIetely effective barrier against sub- trol Association. cause such measures are most effective terranean termites for a period of 18 and economical at that time. Preventive years in heavily infested forest soil in Prevention measures fall into two general categories, Mississippi, and may continue to be effec- Some househoId pests, including cock- depending on whether they are directed tive for many more years. The pest con- roaches, silverfish, odorous house ants, against (1) subterranean termites, which trol industry has successfully used soil plaster beetles (Lathrididae) , psocids, invade a building from colonies in the treatment for many years. and rat mites, spend all or a major part soil and which first attack in the sub- Currently recommended insecticides, of their time in hidden areas of a house structure; or (2) a number of other in- and concentrations include 0.5 per cent such as the attic, wall voids, soffit voids, sect species that spend either all or a aldrin, dieldrin, or heptachlor, or 1 per or in voids under cabinets or built-in considerable part of their life in the attic, cent chlordane.