Toxicological Review of Chlordane (Technical)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -

Prenatal Organochlorine Pesticide Exposure and the Disruption of Steroids and Reproductive Hormones in Cord Blood : Title the Hokkaido Study
Prenatal organochlorine pesticide exposure and the disruption of steroids and reproductive hormones in cord blood : Title The Hokkaido study Araki, Atsuko; Miyashita, Chihiro; Mitsui, Takahiko; Goudarzi, Houman; Mizutani, Futoshi; Chisaki, Youichi; Itoh, Author(s) Sachiko; Sasaki, Seiko; Cho, Kazutoshi; Moriya, Kimihiko; Shinohara, Nobuo; Nonomura, Katsuya; Kishi, Reiko Environment international, 110, 1-13 Citation https://doi.org/10.1016/j.envint.2017.10.006 Issue Date 2018-01 Doc URL http://hdl.handle.net/2115/76437 © 2018. This manuscript version is made available under the CC-BY-NC-ND 4.0 license Rights http://creativecommons.org/licenses/by-nc-nd/4.0/ Rights(URL) http://creativecommons.org/licenses/by-nc-nd/4.0/ Type article (author version) File Information ENVINT_2017_824_(final).pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 2 3 4 5 6 1 Prenatal organochlorine pesticide exposure and the disruption of steroids and 7 8 2 reproductive hormones in cord blood: The Hokkaido Study 9 10 3 Atsuko Arakia, Chihiro Miyashitaa, Takahiko Mitsuib,c, Houman Goudarzia,d, Futoshi 11 12 4 Mizutanie, Youichi Chisakie, Sachiko Itoha, Seiko Sasakif, Kazutoshi Chog, Kimihiko 13 14 5 Moriyah, Nobuo Shinoharah, Katsuya Nonomurah,i, and Reiko Kishia 15 16 6 17 18 7 aCenter for Environmental and Health Sciences, Hokkaido University, Kita 12, Nishi 7, 19 20 8 Sapporo, Hokkaido, Japan 21 22 b 23 9 Department of Urology, Hokkaido University Hospital, Kita 15, Nishi 7, Sapporo, 24 25 10 Hokkaido, Japan 26 -
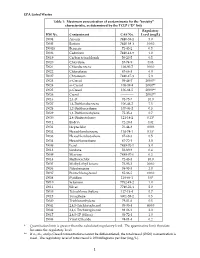
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Health Consultation Chevron Chemical Co
- • ! t Health Consultation Chevron Chemical Co. (Ortho Division) Orlando, Orange County, Florida CERCLIS NO. FLD004064242 June 1995 Prepared by Environmental Toxicology The Florida Department of Health and Rehabilitative Services Under a Cooperative Agreement With Agency for Toxic Substances and Disease Registry U.S. Public Health Service Department of Health and Human Services - ' ' Health Consultation - Chevron Chemical June 1995 Background and Statement of Issues The purpose of this health consultation is to interpret the results of indoor air monitoring for pesticides in two trailers adjacent to the Chevron Chemical Superfund site in Orlando, Florida. During a March 9, 1995 public meeting, two nearby residents expressed concerns that the insides of their trailers were contaminated with pesticides. We agreed to test their trailers for pesticide contamination. The Chevron Chemical Co. (Ortho Division) Superfund site is a former pesticide formulation plant and truck repair facility in Orlando, Florida (Figures 1-3, Appendix A). Past waste disposal practices contaminated soil and ground water. Stormwater run-off carried pesticide-contaminated soil to the adjacent Armstrong Trailer Park. In 1992, the Chevron Chemical Company removed the on-site contaminated soil. In 1994, they removed the contaminated soil from the Armstrong Trailer Park. In a 1995 public health assessment (ATSDR 1995), we found the site was a public health hazard because some residents of the adjacent Armstrong Trailer Park may have unknowingly eaten small amounts of soil contaminated with the pesticide chlordane. As a result, we estimated those residents have a moderately increased risk of liver cancer. Since Chevron cleaned up the chlordane-contaminated soil at this trailer park, we estimated the remaining cancer risk from chlordane is insignificant. -

Chronology of Pesticides Used on National Park Service Collections
Conserve O Gram June 2001 Number 2/16 Chronology Of Pesticides Used On National Park Service Collections The history of National Park Service pesticide use publication). Synonyms and trade names were policy for museum collection objects is obtained from the Merck Index, notes from the documented in various publications including IPM Coordinator, and two Internet sites Field Manual for Museums (Burns), Manual for (<http://chemfinder.com> and <http:// Museums (Lewis), versions of the Museum www.cdpr.ca.gov/cgi-bin/epa>). Handbook, Part I, and two versions of the Integrated Pest Management Information Manual. Not all of the chemicals listed in the Other non-policy sources include Coleman's accompanying charts were marketed as pesticides. Manual for Small Museums, object treatment Some are fungicides and microbiocides. One, reports and notes from NPS staff, and notes from Lexol, is a leather preservative and consolidant. the Office of the Integrated Pest Management All of these are included here because records (IPM) Coordinator. indicate they were applied to objects as pesticides. The two accompanying charts list the types of The potential for pesticide residue remaining on pesticides that may have been used on National collection objects is very high. Objects with such Park Service collections along with some common residues pose a health risk to curatorial staff and synonyms and trade names. to the public who come into physical contact with them, unless proper precautions are taken. Dates shown in blue on the chart represent Additional information on health and safety issues published recommendations for the use of and protective measures can be found in the pesticides. -

Results from Monitoring Endosulfan and Dieldrin in Wide Hollow Creek, Yakima River Drainage, 2005-06
A D e p a r t m e n t o f E c o l o g y R e p o r t Results from Monitoring Endosulfan and Dieldrin in Wide Hollow Creek, Yakima River Drainage, 2005-06 Abstract Endosulfan and dieldrin were monitored in Wide Hollow Creek near Yakima from July 2005 through June 2006. Wide Hollow Creek is on the federal Clean Water Act Section 303(d) list as water quality limited for historically exceeding aquatic life and/or human health water quality criteria for these pesticides. Results showed that endosulfan no longer qualifies for 303(d) listing. Dieldrin, however, was consistently above human health criteria and should therefore remain listed. Data were also obtained on endosulfan sulfate and aldrin. By Art Johnson and Chris Burke Publication No. 06-03-038 September 2006 Waterbody No. WA-37-1047 Publication Information This report is available on the Department of Ecology’s website at www.ecy.wa.gov/biblio/0603038.html Data for this project are available on Ecology’s Environmental Information Management (EIM) website at www.ecy.wa.gov/eim/index.htm. Search User Study ID, AJOH0053. Ecology’s Study Tracker Code for this study is 06-001. For more information contact: Publications Coordinator Environmental Assessment Program P.O. Box 47600 Olympia, WA 98504-7600 E-mail: [email protected] Phone: (360) 407-6764 Authors: Art Johnson and Chris Burke Watershed Ecology Section Environmental Assessment Program Washington State Department of Ecology E-mail: [email protected], [email protected] Phone: 360-407-6766, 360-407-6139 Address: PO Box 47600, Olympia WA 98504-7600 Any use of product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the author or the Department of Ecology. -

Chlordane and Toxaphene Residues Following Cooking of Treated Channel Catfish Fillets
763 Journal of Food Protection, Vol. 63, No. 6, 2000, Pages 763±767 Copyright Q, International Association for Food Protection Chlordane and Toxaphene Residues following Cooking of Treated Channel Cat®sh Fillets C. R. SANTERRE,1* R. INGRAM,2 D. H. XU,3 G. W. LEWIS,4 AND L. G. LANE2 1Department of Foods and Nutrition, Purdue University, West Lafayette, Indiana 47907-1264; 2Mississippi State Chemical Laboratory, Mississippi State, Mississippi 39762; 3Department of Fisheries and Allied Aquacultures, Auburn University, Auburn, Alabama 36849; and 4Warnell School of Forest Resources, University of Georgia, Athens, Georgia 30602, USA MS 99-215: Received 28 July 1999/Accepted 17 December 1999 Downloaded from http://meridian.allenpress.com/jfp/article-pdf/63/6/763/1673844/0362-028x-63_6_763.pdf by guest on 27 September 2021 ABSTRACT The reduction in residues of chlordane and toxaphene following cooking (frying, baking, and smoking) of ®llets obtained from treated Channel cat®sh (Ictalurus punctatus) was determined. On average, cooking reduced moisture content by 17% and increased fat content by 28 to 274%. Frying reduced chlordane residues by 56 to 86% on a dry basis (db) or 84 to 92% on a percent fat basis (fb) when raw ®llets were compared to cooked ®llets. Baking and smoking reduced chlordane signi®cantly less (P , 0.05) than frying with reductions in residues of 12% and 9% (db) or 30% and 33% (fb), respectively. Frying reduced toxaphene residues by 40 to 49% (db) or 65 to 77% (fb), while baking and smoking reduced toxaphene by 35% and 24% (db) or 51% and 59% (fb), respectively. -

TOXICOLOGICAL PROFILE for CHLORDANE U.S. DEPARTMENT of HEALTH and HUMAN SERVICES Public Health Service Agency for Toxic Subs
TOXICOLOGICAL PROFILE FOR CHLORDANE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 1994 CHLORDANE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. CHLORDANE iii UPDATE STATEMENT A Toxicological Profile for Chlordane was released on December 1989. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 CHLORDANE vii CONTRIBUTORS CHEMICAL MANAGERS(S)/AUTHOR(S): Henry G. Abadin, M.S.P.H. ATSDR, Division of Toxicology, Atlanta, GA Ronald Baynes, D.V.M., M.S. Paul F. Goetchius, D.V.M. Syracuse Research Corporation, Syracuse, NY THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying endpoints. 3 . Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. 4 . Quality Assurance Review. The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problems in format or content, and establishes that Guidance has been followed. -

Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery
COMMON HOUSEHOLD HAZARDOUS WASTES CORROSIVES (ACIDS) Glues POISONS Boric Acid Grease Ant and Roach Killer Car Battery Acid Household Waxes Arsinic Compounds Copper Cleaners Isopropyl Alcohol Automotive Cleaners Etching Solutions Kerosene Bacterial Pipe Cleaners Ferric Chloride Lacquer Thiner Bordeaux Mix Hydrochloric Acid Lacquer Paint (unsolidified) Boric Acid Hydrofluoric Acid Linseed Oil Bug Removers Metal Cleaners Liquid Waxes Chlordane Muriatic Acid Liquid Sandpaper Chromium Navel Jelly Liquid Butane Copper Sulfate Phosphoric Acid Methanol DDT Pool Acid Naphtha Diazinon Sodium Bisulfate Oils (petroleum) Dimethylamine Salts Sulfuric Acid Organic Solvents Disinfectants Toilet Bowl Cleaners Paint Thinners Dog Repellent Paint Strippers Fertilizers CORROSIVES (BASES) Paraffin Oil Flea Spray/Powders Ammonia Perfume Fluorine Ammonia Based Cleaners Petroleum Distillers Fungicides Battery Terminal Cleaners Plastic Roof Cement Gopher Killer Caustic Soda Plastic Model Cement Hydrofluoric Acid Cess Pool Cleaners Polyurethane Paint (unsolidified) Insect Sprays Drain Cleaners Polyurethane Cement (unsolid.) Lead Compounds Household Cleaners Powe Steering Fluid Lice Powders Lime Primers Lindane Lye Roofing Cement Malathion Oven Cleaners Rug/Upholstery Cleaner Methylene Chloride Sodium Hydroxide Sealers Mole Killer Window Cleaners Shellac Thinner Moth Crystals Silicone Sprays Pesticides FLAMMABLES & COMBUSTIBLES Spot Remover/Dry Clean Fluids Pharmaceuticals Acetone Thinner Plant Food Adhesives Tile Cement Pruning Paint Aerosol Tire Black Pyethrins -

Table 1 Summary of Soil Analytical Methods
TABLE 1 SUMMARY OF SOIL ANALYTICAL METHODS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California No. of Analyzed Compound EPA Method Soil Samples Organochlorine Pesticides (OCPs) EPA Method 8081A 26 Total Lead EPA Method 6010B 30 Arsenic EPA Method 6010B 73 Page 1 of 1 TABLE 2 SUMMARY OF SOIL ANALYTICAL RESULTS CRENSHAW HIGH SCHOOL 5010 11th Avenue Los Angeles, California Number. of Number of Analyzed Range of Analyzed Samples with Compounds Detections Samples Detections Organochlorine Pesticides 26 2 2-26 ug/kg a-chlordane 26 2 both 5 ug/kg d-chlordane 26 2 3-4 ug/kg total chlordane 26 2 11-26 ug/kg dieldrin 26 1 2 ug/kg Total Lead 30 29 1-53 mg/kg Arsenic 73 46 1-34 mg/kg mg/kg - milligrams per kilogram ug/kg - micrograms per kilogram mdl - method detection limit Page 1 of 1 TABLE 3 SUMMARY OF SOIL ANALYTICAL RESULTS PESTICIDES CRENSHAW HIGH SCHOOL 5010 11the Avenue Los Angeles, California Compound PB-2-6" PB-3-6" PB-5-6" PB-8-6" PB-12-6" PB-13-6" PB-18-6" EPA Method 8081A (ug/kg) Aldrin ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-BHC (Lindane) ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 beta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 delta-BHC ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 alpha-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 gamma-Chlordane ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 Total Chlordane ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 ND < 5 4,4'-DDD ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 ND < 1 -

QSAR Model for Androgen Receptor Antagonism
s & H oid orm er o t n S f a l o S l c a Journal of i n e Jensen et al., J Steroids Horm Sci 2012, S:2 r n u c o e DOI: 10.4172/2157-7536.S2-006 J ISSN: 2157-7536 Steroids & Hormonal Science Research Article Open Access QSAR Model for Androgen Receptor Antagonism - Data from CHO Cell Reporter Gene Assays Gunde Egeskov Jensen*, Nikolai Georgiev Nikolov, Karin Dreisig, Anne Marie Vinggaard and Jay Russel Niemelä National Food Institute, Technical University of Denmark, Department of Toxicology and Risk Assessment, Mørkhøj Bygade 19, 2860 Søborg, Denmark Abstract For the development of QSAR models for Androgen Receptor (AR) antagonism, a training set based on reporter gene data from Chinese hamster ovary (CHO) cells was constructed. The training set is composed of data from the literature as well as new data for 51 cardiovascular drugs screened for AR antagonism in our laboratory. The data set represents a wide range of chemical structures and various functions. Twelve percent of the screened drugs were AR antagonisms; three out of six statins showed AR antagonism, two showed cytotoxicity and one was negative. The newly identified AR antagonisms are: Lovastatin, Simvastatin, Mevastatin, Amiodaron, Docosahexaenoic acid and Dilazep. A total of 874 (231 positive, 643 negative) chemicals constitute the training set for the model. The Case Ultra expert system was used to construct the QSAR model. The model was cross-validated (leave-groups-out) with a concordance of 78.4%, a specificity of 86.1% and a sensitivity of 57.9%.