Giant-Planet Chemistry: Ammonium Hydrosulfide (NH4SH), Its IR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
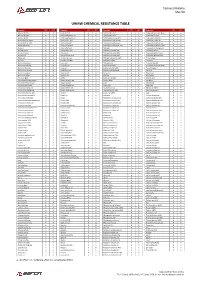
Chemical Properties
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -
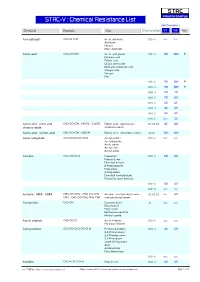
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Massachusetts Oil and Hazardous Material List
SUBPART P: MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST TABLE OF CONTENTS TABLE 1 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (ALPHABETICAL LISTING) TABLE 2 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (BY CAS NUMBER ORDER) NOTES: The Massachusetts Oil and Hazardous Materials List (MOHML) contains oils and hazardous materials subject to 310 CMR 40.0000 and their reportable quantities (RQs) and reportable concentrations (RCs). These values are referred to in the notification requirements (310 CMR 40.0300). This list is provided both alphabetically in Table 1 and by Chemical Abstracts Service Number (CAS Number) in Table 2. The CAS number is a unique number assigned to a substance. Both tables identify other lists on which a substance appears by using name source codes. These codes are as follows: Name Source 1 - The Department of Transportation (DOT) Hazardous Materials List (49 CFR Part 172.101 Hazardous Materials Table) Name Source 2 - The Resource Conservation and Recovery Act Appendix VIII List (40 CFR Part 261 - Appendix VIII Hazardous Constituents) Name Source 3 - The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Hazardous Substance and Waste Stream Lists (40 CFR Part 302 - Table 302.4) Name Source 4 - The Extremely Hazardous Substance List as mandated by Superfund Amendments and Reauthorization Act, Title III, Section 302 (40 CFR Part 355 Appendices A and B) Name Source 5 - DEP Allowable Ambient Limits (AALs) and Drinking Water Guidelines Name Source 6 - The Massachusetts Substance List (MSL)(105 CMR 670.000: “Right to Know” Appendix A) Name Source 7 - The Chemical Abstracts name, 9th collective period, 1972-1976 Name Source 8 - The EPA Right to Know list, Section 313 of the Emergency Planning and Community Right to Know Act of 1986 (40 CFR Part 372.65). -

ICES-2021-292.Pdf (1.890Mb)
50th International Conference on Environmental Systems ICES-2021-292 12-15 July 2021 Experimental Proof of Concept of a Cold Trap as a Purification Step for Lunar Water Processing Jordan B. Holquist1, Sean Gellenbeck2, Chad E. Bower3, and Philipp Tewes4 Paragon Space Development Corporation, Tucson, AZ 85714, USA Since the observation of direct evidence of water-ice in the permanently shadowed regions (PSR) on the lunar surface, in-situ resource utilization (ISRU) has been proposed for processing the regolith-bound water-ice to provide fresh water, breathable oxygen, and liquid rocket propellant for lunar exploration missions. One possible method of extraction is the sublimation and vapor transport of the water from the regolith to a collection and processing system. However, the water-ice is found concurrently with other typically volatile species that can sublimate with water vapor and that would contaminate and degrade downstream processing systems. Paragon Space Development Corporation® is developing the ISRU- derived water purification and Hydrogen Oxygen Production (IHOP) system to collect, purify, and process water-ice from PSRs on the lunar surface. A critical component of this system concept is a cold trap that selectively deposits water-ice from a saturated water vapor stream while rejecting the contaminant volatiles. This paper presents results, analysis, and discussion of an experimental proof of concept demonstration wherein a warm process gas containing water vapor and volatile contaminant components (H2, CO, H2S, NH3, SO2, C2H4, CH4, CO2, and CH3OH) was passed into an evacuated and actively chilled thick-walled glass bottle. Water-ice samples were collected from each of eight test batches with varying contaminant components present at concentrations with respect to water vapor matching their observed proportionality from the LCROSS mission results. -

United States Patent (19) 3,728,439 Urban |45 Apr
United States Patent (19) 3,728,439 Urban |45 Apr. 17, 1973 54 TREATMENT OF A SULFTE Primary Examiner - Oscar R. Vertiz CONTAINING AOUEOUS STEAM TO Assistant Examiner-George O. Peters SELECTIVELY PRODUCE Attorney-James R. Hoatson, Jr. and Thomas K. Mc ELEMENTAL SULFUR Bride 75) Inventor: Peter Urban, Northbrook, Ill. 57 ABSTRACT (73) Assignee: Universal Oil Products Company, An input water stream containing a water-soluble Des Plaines, Ill. sulfite compound is treated to produce elemental sul 22 Filed: Nov. 5, 1970 fur, while minimizing the formation of undesired sulfate by-products by the steps of: (a) selectively con (21) Appl. No.: 87,393 verting the sulfite compound to the corresponding thiosulfate compound; (b) reacting a portion of the (52) U.S. C. .....................423.1567,210/50, 4231571 resulting thiosulfate-containing stream with a reducing (51) Int. Cl. .............................................C01b 17/02 agent to form a sulfide-containing stream; (c) reacting 58 Field of Search.................. 23/224, 229; 210/50, the remaining portion of the thiosulfate-containing 20/63 stream with the sulfide-containing stream to form the corresponding polysulfide compound; and thereafter, subjecting the resulting stream containing the polysul (56) References Cited fide compound to decomposition conditions selected UNITED STATES PATENTS to form elemental sulfur, hydrogen sulfide, and a sub 1,636,106 7/1927 Naef.......................................23/137 stantially sulfate-free treated water stream. The prin cipal utility of this treatment procedure is associated 3,536,618 Of 1970 Urban et al..... ...23/224 X with the clean up, or regeneration, of sulfite-contain 1,062,120 5/1913 Sanborn................................ -

New York City Department of Environmental Protection Community Right-To-Know: List of Hazardous Substances
New York City Department of Environmental Protection Community Right-to-Know: List of Hazardous Substances Updated: 12/2015 Definitions SARA = The federal Superfund Amendments and Reauthorization Act (enacted in 1986). Title III of SARA, known as the Emergency Planning and Community Right-to-Act, sets requirements for hazardous chemicals, improves the public’s access to information on chemical hazards in their community, and establishes reporting responsibilities for facilities that store, use, and/or release hazardous chemicals. RQ = Reportable Quantity. An amount entered in this column indicates the substance may be reportable under §304 of SARA Title III. Amount is in pounds, a "K" represents 1,000 pounds. An asterisk following the Reporting Quantity (i.e. 5000*) will indicate that reporting of releases is not required if the diameter of the pieces of the solid metal released is equal to or exceeds 100 micrometers (0.004 inches). TPQ = Threshold Planning Quantity. An amount entered in this column reads in pounds and indicates the substance is an Extremely Hazardous Substance (EHS), and may require reporting under sections 302, 304 & 312 of SARA Title III. A TPQ with a slash (/) indicates a "split" TPQ. The number to the left of the slash is the substance's TPQ only if the substance is present in the form of a fine powder (particle size less than 100 microns), molten or in solution, or reacts with water (NFPA rating = 2, 3 or 4). The TPQ is 10,000 lb if the substance is present in other forms. A star (*) in the 313 column= The substance is reportable under §313 of SARA Title III. -
United States Patent Office Patented Jan
3,489,677 United States Patent Office Patented Jan. 13, 1970 2 oil and fractions thereof with a reactive aqueous solution 3,489,677 REMOVAL OF ELEMENTAL SULFUR CON capable of extracting from the oil any entrained or dis TAMENANTS FROM PETROLEUM OLS Solved elemental sulfur present therein. William H. Thompson, Lafayette, and Eldred E. Young, Other objects will become apparent during the follow Concord, Calif., assignors to Shell Oil Company, New ing discussion of the invention. York, N.Y., a corporation of Delaware No Drawing. Filed Nov. 20, 1967, Ser. No. 684,507 SUMMARY OF THE INVENTION Ent. C. C10g 29/02 Now in accordance with the present invention it has U.S. C. 208-236 11. Claims been found that elemental sulfur can be separated effec O tively from crude oil and fractions thereof containing less than about 5%, and generally between about 0.5% ABSTRACT OF THE DISCLOSURE and 1.5% of elemental sulfur, by contacting such con A method of purifying elemental sulfur contaminated taminated oils with an aqueous solution containing a oils by treating such elemental sulfur contaminated oils mixture of an alkali hydrosulfide and hydroxide such as with an aqueous solution containing a mixture of alkali 5 ammonium hydrosulfide and ammonium hydroxide for a hydrosulfide and corresponding hydroxide, e.g., ammo period of time and under controlled temperature condi nium hydrosulfide and ammonium hydroxide. tions to effect a reaction between the elemental sulfur dispersed or solubilized in 1he oil and the alkali com pounds present in the aqueous solution so that on sepa The present invention relates to a new and novel proc 20 ration of the oil phase from the aqueous phase, the sulfur ess for removing elemental sulfur from elemental sulfur has been transferred in the latter phase where it is pres contaminated petroleum oils. -
Recovery of Sulfur from Phosphogypsum: Conversion of Calcium Sulfide to Sulfur
PLEASE DO Nar REMOVE FROVI LIBRARY Recovery of Sulfur From Phosphogypsum: Conversion of Calcium Sulfide to Sulfur By David A. Rice, Olice C. Carter, Jr., Alexander May, Margaret M. Ragin, and Robert G. Swanton 1910 * 80 * 1990 YEARS AU OF MI UNITED STATES DEPARTMENT OF THE INTERI U.S. Bureau of Mine~ ··"1 Spo!(;:ifle Research Center E. :;! 5 Montgomery twe. Sn':1:~:na, INA 99207 i F? [il Lb!G:w"hnP h'Y Mission: As the Nation's principal conservation agency, the Department of the Interior has respon sibility for most of our nationally-owned public lands and natural and cultural resources. This includes fostering wise use of our land and water resources, protecting our fish and wildlife, pre serving the environmental and cultural values of our national parks and historical places, and pro viding for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interests of all our people. The Department also promotes the goals of the Take Pride in America campaign by encouraging stewardship and citizen responsibil ity for the public lands and promoting citizen par ticipation in their care. The Department also has a major responsibility for American Indian reser vation communities and for people who live in Island Territories under U.S. Administration. 1 i I ! Report of Investigations 9297 Recovery of Sulfur From Phosphogypsum: Conversion of Calcium Sulfide to Sulfur By David A. Rice, Olice C. Carter, Jr., Alexander May, Margaret M. Ragin, and Robert G. -
Hazardous Waste Management Guide
Hazardous Waste Management Guide Touro University California The university community plays a vital role in the management of hazardous wastes on our campus. Proper waste management is dependent upon your day-to-day handling of these wastes in your lab or worksite. Please read the Guide carefully. Last Updated: May 11, 2010 1 CONTACT INFORMATION Campus Emergency/Fire/Police (from a campus phone) 9-911 Vallejo Fire Department (non-emergency) 707-638-4526 Vallejo Police Department (non-emergency) 707-638-4321 Ambulance 707-552-1193 Campus Security (from a campus phone) 8-5804 Campus Facilities (from a campus phone) 8-5800 Campus Facilities (pager- after business hour) 707-551-6034 Biosafety Officer (from a campus phone) 8-5239 2 TABLE OF CONTENT SECTIONS INTRODUCTION.................................................................................................... 4 HAZARDOUS WASTE........................................................................................... 5 REQUIREMENTS FOR CHEMICAL WASTE...................................................... 5 CLASSIFICATION OF CHEMICAL WASTE....................................................... 7 GENERAL LABELING & PACKAGING …………………................................. 8 SPECIFIC LABELING & PACKAGING…………………................................... 9 DISPOSAL OF UNKNOWNS................................................................................. 15 BIOHAZARDOUS WASTE.................................................................................... 15 General Labeling, Packaging & Disposal -

United States Patent Office Patented Oct
3,281,355 United States Patent Office Patented Oct. 25, 1966 2 MoC)Br2, MoaOCls, MoOCls, MoOF. It is also pos 3,281,355 sible to employ aqueous sulfuric acid solutions of MoC3, STABLE COLLOIDAL DESPERSONS OF or H2MoC) or molybdenum blue. Other suitable start MOLYBDENUM SULFIDE ing materials include solutions of molybdenum naph Elmer B. Cyphers, Cranford, and Max W. Hill, Westfield, N.J., assignors to Esso Research and Engineering Com thenates or sulfonates in one or more of the lower alco pany, a corporation of Delaware hols. By lower alcohol is meant a C to Caliphatic al No Drawing. Filed Nov. 15, 1963, Ser. No. 323,887 cohol, i.e., methanol, ethanol, isopropyl alcohol or butyl 14 Claims. (Cl. 252-18) alcohol. The formation of the molybdenum sulfide can be car The present application is a continuation-in-part of ap O ried out in the presence of the detergent additive per se. plication Serial No. 99,688, filed March 31, 1961, and It is usually more convenient, however, to carry out the abandoned subsequent to the filing of the present appli formation in an oil concentrate of the detergent additive. cation. These concentrates usually contain about 10 to 80 wt. This invention relates to the preparation of composi percent of the detergent additive, with the remainder be tions containing stably dispersed molybdenum sulfide of 5 ing lubricating oil. colloidal particle size and to the use of such compositions The lubricating oil may be either a mineral oil or a in lubricating oil formulations. Particularly, the inven synthetic oil.