List/Code of Hazardous Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
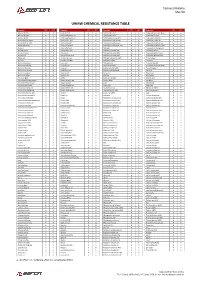
Chemical Properties
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION
T-6 Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION, AND THE MOLE FORMULAS: A chemical formula shows the elemental composition of a substance: the chemical symbols show what elements are present and the numerical subscripts show how many atoms of each element there are in a formula unit. Examples: NaCl: one sodium atom, one chlorine atom in a formula unit CaCl2: one calcium atom, two chlorine atoms in a formula unit Mg3N2: three magnesium atoms, two nitrogen atoms in a formula unit The presence of a metal in a chemical formula indicates an ionic compound, which is composed of positive ions (cations) and negative ions (anions). A formula with only nonmetals indicates a + molecular compound (unless it is an ammonium, NH4 , compound). Only ionic compounds are considered in this Tutorial. There are tables of common ions in your lecture text, p 56 (cations) and p 57 (anions). A combined table of these same ions can be found on the inside back cover of the lecture text. A similar list is on the next page; all formulas needed in this and subsequent Tutorial problems can be written with ions from this list. Writing formulas for ionic compounds is very straightforward: TOTAL POSITIVE CHARGES MUST BE THE SAME AS TOTAL NEGATIVE CHARGES. The formula must be neutral. The positive ion is written first in the formula and the name of the compound is the two ion names. EXAMPLE: Write the formula for potassium chloride. The name tells you there are potassium, K+, and chloride, Cl–, ions. Each potassium ion is +1 and each chloride ion is -1: one of each is needed, and the formula for potassium chloride is KCl. -

Inventory Size (Ml Or G) 103220 Dimethyl Sulfate 77-78-1 500 Ml
Inventory Bottle Size Number Name CAS# (mL or g) Room # Location 103220 Dimethyl sulfate 77-78-1 500 ml 3222 A-1 Benzonitrile 100-47-0 100ml 3222 A-1 Tin(IV)chloride 1.0 M in DCM 7676-78-8 100ml 3222 A-1 103713 Acetic Anhydride 108-24-7 500ml 3222 A2 103714 Sulfuric acid, fuming 9014-95-7 500g 3222 A2 103723 Phosphorus tribromide 7789-60-8 100g 3222 A2 103724 Trifluoroacetic acid 76-05-1 100g 3222 A2 101342 Succinyl chloride 543-20-4 3222 A2 100069 Chloroacetyl chloride 79-04-9 100ml 3222 A2 10002 Chloroacetyl chloride 79-04-9 100ml 3222 A2 101134 Acetyl chloride 75-36-5 500g 3222 A2 103721 Ethyl chlorooxoacetate 4755-77-5 100g 3222 A2 100423 Titanium(IV) chloride solution 7550-45-0 100ml 3222 A2 103877 Acetic Anhydride 108-24-7 1L 3222 A3 103874 Polyphosphoric acid 8017-16-1 1kg 3222 A3 103695 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103694 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103880 Methanesulfonic acid 75-75-2 500ml 3222 A3 103883 Oxalyl chloride 79-37-8 100ml 3222 A3 103889 Thiodiglycolic acid 123-93-3 500g 3222 A3 103888 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 103886 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 102969 sulfuric acid 7664-93-9 500 mL 2428 A7 102970 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102971 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102973 formic acid (88%) 64-18-6 500 mL 2428 A7 102974 hydrofloric acid (49%) 7664-39-3 500 mL 2428 A7 103320 Ammonium Hydroxide conc. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara Et Al
US 20110027386A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara et al. (43) Pub. Date: Feb. 3, 2011 (54) ANTMICROBAL. ZEOLITE AND (30) Foreign Application Priority Data ANTMICROBAL COMPOSITION Feb. 22, 2006 (JP) ................................. 2006-045241 (75) Inventors: Yasuo Kurihara, Nagoya-shi (JP); Kumiko Miyake, Nagoya-shi (JP); Publication Classification Masashi Uchida, Nagoya-shi (JP) (51) Int. Cl. Correspondence Address: AOIN 59/6 (2006.01) NIXON & VANDERHYE, PC COB 39/02 (2006.01) 901 NORTH GLEBE ROAD, 11TH FLOOR AOIP I/00 (2006.01) ARLINGTON, VA 22203 (US) (52) U.S. Cl. .......................... 424/618; 423/701; 423/700 (73) Assignee: Sinanen Zeomic Co., Ltd., (57) ABSTRACT Nagoya-Shi (JP) The present invention relates to antimicrobial zeolite which comprises zeolite whereina hardly soluble zinc salt is formed (21) Appl. No.: 12/923,854 within fine pores present therein and an antimicrobial com position which comprises the foregoing antimicrobial Zeolite (22) Filed: Oct. 12, 2010 in an amount ranging from 0.05 to 80% by mass. The antimi crobial Zeolite according to the present invention can widely Related U.S. Application Data be applied, without causing any color change, even to the (63) Continuation of application No. 1 1/705,460, filed on goods which undergo color changes with the elapse of time Feb. 13, 2007. when the conventional antimicrobial zeolite is added. US 2011/002738.6 A1 Feb. 3, 2011 ANTMICROBAL. ZEOLITE AND 3. An antimicrobial composition comprising the foregoing ANTMICROBAL COMPOSITION antimicrobial zeolite as set forth in the foregoing item 1 or 2 in an amount ranging from 0.05 to 80% by mass. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -

PRICELIST-1920-FINAL.Pdf
INDEX Page No. MD Speech 01 Our Vision / Our Mission 02 Product Classification and Grade Information 03 Label Information 04 GHS Compliance 05 Technical Data Sheet and COA 06 Qualikems Product Range 07 ISO Certificate 08 - 09 Company Details 10 Ordering Information 11 Terms & Conditions 12 Rate List 13 - 52 Images of Lab / Plant / R & D 53 - 58 Rate List 59 -116 BELIEVING yourselfIN IS THE FIRST SECRET TO Success Dear Reader, The document you are holding is the result of work performed by the team of professionals of QUALIKEMS. It is the fruit of our teams extensive technical experience combine with the collaboration of our customers, who have offered us their valuable comments and proposals for improvement. At Qualikems, we have been working and investing for many years with our thoughts focused on the long term. Only thus can this comprehensive catalogue be kept up to date with the products you need. Our highly trained workforce, using state of the art technology, is the driving force behind the management of our modern factory, and our principal aim is to guarantee that the QUALIKEMS product range meets the conditions you require. QUALIKEMS reinforces industrial character and the path to progress we have continuously forged over the years. This path requires the responsible use of resources and the sustainability of our business activity. It is likewise requires and ability to keep on growing as the way to earn and to preserve our status as the leading supplier of laboratory reagents to our Clients Ashok Sahni Managing Director QUALIKEMS FINE CHEM PVT. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

United States Patent (19) 11 Patent Number: 5,883,058 Wells Et Al
USOO5883058A United States Patent (19) 11 Patent Number: 5,883,058 Wells et al. (45) Date of Patent: *Mar 16, 1999 54 HIGH LATHER STYLING SHAMPOOS 4,784,801 11/1988 Hoeffkes et al. ....................... 252/554 5,084.212 1/1992 Farris et al. ............................ 252/554 (75) Inventors: Robert Lee Wells, Cincinnati, Ohio; 5,104,642 4/1992 Wells et al. ..... ... 424/47 Jon Robert Behrens, Kobe, Japan 5,120,532 6/1992 Wells et al. ............ ... 424/70 5,310,508 5/1994 Subramanyam et al. ............... 252/549 73) Assignee: The Procter & Gamble Company, 5,391,368 2/1995 Gerstein ............................... 424/70.13 5,514,302 5/1996 Brown ..................................... 252/545 Cincinnati, Ohio 5,580,494 12/1996 Sandhu et al. .......................... 510/125 Notice: The term of this patent shall not extend FOREIGN PATENT DOCUMENTS beyond the expiration date of Pat. No. 5,672.576. 0323715 12/1989 European Pat. Off.. Appl. No.: 520,631 Primary Examiner Paul Lieberman Assistant Examiner Necholas Ogden Filed: Aug. 29, 1995 Attorney, Agent, or Firm Joan B. Tucker; William J. Int. Cl." ................................................ C110 1/83 Winter; Tara M. Rosnell U.S. Cl. .......................... 510/127; 510/119,510/123; 57 ABSTRACT 510/125; 424/70.11; 424/70.24 The present invention relates to hair shampoo compositions Field of Search ..................................... 252/549, 550, which have improved cleansing, lathering, and Styling ben 252/551, 557; 510/119, 123,125, 127; efits=. These Shampoo compositions comprise an alkyl glyc 424/70.24, 70.11 eryl ether Sulfonate Surfactant, a hair Styling polymer, a 56) References Cited non-polar volatile Solvent, and water. -

Ammonium Formate As Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-Ααα-Methylbenzylamine
RSC Advances Ammonium Formate as Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-ααα-Methylbenzylamine Journal: RSC Advances Manuscript ID: RA-ART-01-2014-000462.R1 Article Type: Paper Date Submitted by the Author: 21-Feb-2014 Complete List of Authors: Miranda, Leandro S. M.; Federal University of Rio de Janeiro, Biocatalysis and Organic Synthesis Lab, Chemistry Institute de Souza, Rodrigo Octavio; Federal University of Rio de Janeiro, de Miranda, Amanda; Federal University of Rio de Janeiro, Page 1 of 21 RSC Advances Graphical Abstract RSC Advances Page 2 of 21 Ammonium Formate as Green Hydrogen Source for Clean Semi-Continuous Enzymatic Dynamic Kinetic Resolution of (+/-)-α- Methylbenzylamine Amanda S. de Miranda, [a] Rodrigo O. M. A. de Souza, [ a] Leandro S. M. Miranda [a]* Keywords: Dynamic kinetic resolution • racemic amines • continuous flow . ammonium formate. Abstract: Abstract: The chemoenzymatic dynamic kinetic resolution of (+/-)-α- Methylbenzylamine under continuous flow conditions in the presence of Pd/BaSO 4 as racemization catalyst and ammonium formate as reductant is described. Under the conditions developed good conversions and excellent enantiomeric excess are reported Page 3 of 21 RSC Advances Introduction Recently, continuous processing and biocatalysis have been elected as key green engineering research areas for sustainable manufacturing 1a and it is clear that joint efforts between these areas can lead to great improvements on continuous manufacturing in agreement with green chemistry principles 1b,c . Optically pure amines are ubiquitously present in nature and active pharmaceutical ingredients (APIs). However, their synthesis still represents an ongoing synthetic challenge that can be inferred by the great amount of work and methodologies dealing with this issue in the literature. -

Review Memorandum
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k062165 B. Purpose for Submission: New device C. Measurand: Barbiturates D. Type of Test: Qualitative and semi-quantitative enzyme immunoassay E. Applicant: Ortho-Clinical Diagnostics, Inc. F. Proprietary and Established Names: VITROS Chemistry Products BARB Reagent VITROS Chemistry Products Calibrator 26 VITROS Chemistry Products FS Calibrator 1 VITROS Chemistry Products DAT Performance Verifiers I, II, III, IV and V G. Regulatory Information: 1. Regulation section: 21 CFR 862.3150, Barbiturates test system 21 CFR 862.3200, Clinical Toxicology Calibrator 21 CFR 862.3180, Clinical Toxicology Control 2. Classification: Class II, (reagent, calibrator) Class I, reserved (control) 3. Product code: DIS, DLJ and DIF 4. Panel: Toxicology (91) 1 H. Intended Use: 1. Intended use(s): See Indications for use. 2. Indication(s) for use: VITROS Chemistry Products BARB Reagent: For in vitro diagnostic use only. VITROS Chemistry Products BARB Reagent is used on VITROS 5,1 FS Chemistry Systems for the semi- quantitative or qualitative determination of barbiturates (BARB) in human urine using a cutoff of 200 ng/mL or 300 ng/mL. Measurements obtained with the VITROS BARB method are used in the diagnosis and treatment of barbiturates use or overdose. The VITROS Chemistry Products BARB assay is intended for use by professional laboratory personnel. It provides only a preliminary test result. A more specific alternative chemical method must be used to confirm a result with this assay. Gas Chromatograpy/Mass Spectrometry (GC/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be applied to any drug-of-abuse test result, particularly when evaluating a preliminary positive result. -

Mechanochemical Catalytic Transfer Hydrogenation of Aromatic Nitro Derivatives
Article Mechanochemical Catalytic Transfer Hydrogenation of Aromatic Nitro Derivatives Tomislav Portada, Davor Margetić and Vjekoslav Štrukil * Division of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia; [email protected] (T.P.); [email protected] (D.M.) * Correspondence: [email protected]; Tel.: +385‐1‐468‐0197 Received: 15 November 2018; Accepted: 29 November 2018; Published: date Abstract: Mechanochemical ball milling catalytic transfer hydrogenation (CTH) of aromatic nitro compounds using readily available and cheap ammonium formate as the hydrogen source is demonstrated as a simple, facile and clean approach for the synthesis of substituted anilines and selected pharmaceutically relevant compounds. The scope of mechanochemical CTH is broad, as the reduction conditions tolerate various functionalities, for example nitro, amino, hydroxy, carbonyl, amide, urea, amino acid and heterocyclic. The presented methodology was also successfully integrated with other types of chemical reactions previously carried out mechanochemically, such as amide bond formation by coupling amines with acyl chlorides or anhydrides and click‐type coupling reactions between amines and iso(thio)cyanates. In this way, we showed that active pharmaceutical ingredients Procainamide and Paracetamol could be synthesized from the respective nitro‐precursors on milligram and gram scale in excellent isolated yields. Keywords: mechanochemistry; catalytic transfer hydrogenation; aromatic nitro derivatives; ammonium formate; aging; ball milling; synthesis 1. Introduction Catalytic hydrogenation is one of the most significant functional group transformation reactions in organic synthesis and numerous procedures and reagents have been developed for that purpose [1,2]. As such, the hydrogenation reaction plays one of the key roles in many industrially important processes, for example hydrogenation of carbon monoxide to methanol or in food industry for the conversion of unsaturated vegetable oils into saturated triglycerides [3].