Furan Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inventory Size (Ml Or G) 103220 Dimethyl Sulfate 77-78-1 500 Ml
Inventory Bottle Size Number Name CAS# (mL or g) Room # Location 103220 Dimethyl sulfate 77-78-1 500 ml 3222 A-1 Benzonitrile 100-47-0 100ml 3222 A-1 Tin(IV)chloride 1.0 M in DCM 7676-78-8 100ml 3222 A-1 103713 Acetic Anhydride 108-24-7 500ml 3222 A2 103714 Sulfuric acid, fuming 9014-95-7 500g 3222 A2 103723 Phosphorus tribromide 7789-60-8 100g 3222 A2 103724 Trifluoroacetic acid 76-05-1 100g 3222 A2 101342 Succinyl chloride 543-20-4 3222 A2 100069 Chloroacetyl chloride 79-04-9 100ml 3222 A2 10002 Chloroacetyl chloride 79-04-9 100ml 3222 A2 101134 Acetyl chloride 75-36-5 500g 3222 A2 103721 Ethyl chlorooxoacetate 4755-77-5 100g 3222 A2 100423 Titanium(IV) chloride solution 7550-45-0 100ml 3222 A2 103877 Acetic Anhydride 108-24-7 1L 3222 A3 103874 Polyphosphoric acid 8017-16-1 1kg 3222 A3 103695 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103694 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103880 Methanesulfonic acid 75-75-2 500ml 3222 A3 103883 Oxalyl chloride 79-37-8 100ml 3222 A3 103889 Thiodiglycolic acid 123-93-3 500g 3222 A3 103888 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 103886 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 102969 sulfuric acid 7664-93-9 500 mL 2428 A7 102970 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102971 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102973 formic acid (88%) 64-18-6 500 mL 2428 A7 102974 hydrofloric acid (49%) 7664-39-3 500 mL 2428 A7 103320 Ammonium Hydroxide conc. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -

Production of Malonic Acid Through the Fermentation of Glucose
University of Pennsylvania ScholarlyCommons Department of Chemical & Biomolecular Senior Design Reports (CBE) Engineering 4-20-2018 Production of Malonic Acid through the Fermentation of Glucose Emily P. Peters University of Pennsylvania, [email protected] Gabrielle J. Schlakman University of Pennsylvania, [email protected] Elise N. Yang University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/cbe_sdr Part of the Biochemical and Biomolecular Engineering Commons Peters, Emily P.; Schlakman, Gabrielle J.; and Yang, Elise N., "Production of Malonic Acid through the Fermentation of Glucose" (2018). Senior Design Reports (CBE). 107. https://repository.upenn.edu/cbe_sdr/107 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/cbe_sdr/107 For more information, please contact [email protected]. Production of Malonic Acid through the Fermentation of Glucose Abstract The overall process to produce malonic acid has not drastically changed in the past 50 years. The current process is damaging to the environment and costly, requiring high market prices. Lygos, Inc., a lab in Berkeley, California, has published a patent describing a way to produce malonic acid through the biological fermentation of genetically modified easty cells. This proposed technology is appealing as it is both better for the environment and economically friendly. For the process discussed in this report, genetically modified Pichia Kudriavzevii yeast cells will be purchased from the Lygos lab along with the negotiation of exclusive licensing rights to the technology. The cells will be grown in fermentation vessels, while being constantly fed oxygen, glucose and fermentation media. The cells will excrete malonic acid in the 101 hour fermentation process. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

(2H, 13C, 15N, 17O and 18O) in Genetically Encoded Amino Acids
Molecules 2013, 18, 482-519; doi:10.3390/molecules18010482 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Access to Any Site Directed Stable Isotope (2H, 13C, 15N, 17O and 18O) in Genetically Encoded Amino Acids Prativa B. S. Dawadi and Johan Lugtenburg * Leiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA, Leiden, The Netherlands; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 25 October 2012; in revised form: 10 December 2012 / Accepted: 24 December 2012 / Published: 2 January 2013 Abstract: Proteins and peptides play a preeminent role in the processes of living cells. The only way to study structure-function relationships of a protein at the atomic level without any perturbation is by using non-invasive isotope sensitive techniques with site-directed stable isotope incorporation at a predetermined amino acid residue in the protein chain. The method can be extended to study the protein chain tagged with stable isotope enriched amino acid residues at any position or combinations of positions in the system. In order to access these studies synthetic methods to prepare any possible isotopologue and isotopomer of the 22 genetically encoded amino acids have to be available. In this paper the synthetic schemes and the stable isotope enriched building blocks that are available via commercially available stable isotope enriched starting materials are described. Keywords: amino acids; isotope labelling; [5-13C]-leucine; [4-13C]-valine; 13C or 15N-enriched L-lysine; [18O]-benzylchloromethyl ether; [13C]-benzonitrile 1. Introduction Proteins and peptides play a preeminent role in living cells, such as receptor action, enzyme catalysis, transport and storage, hormone action, mechanical support, immune protection, etc. -

Exhibit 2D-3
Exhibit 2D–3. Hazardous Substances 1. Acetaldehyde 73. Captan 144. Ferrous sulfate 2. Acetic acid 74. Carbaryl 145. Formaldehyde 3. Acetic anhydride 75. Carbofuran 146. Formic acid 4. Acetone cyanohydrin 76. Carbon disulfide 147. Fumaric acid 5. Acetyl bromide 77. Carbon tetrachloride 148. Furfural 6. Acetyl chloride 78. Chlordane 149. Guthion 7. Acrolein 79. Chlorine 150. Heptachlor 8. Acrylonitrile 80. Chlorobenzene 151. Hexachlorocyclopentadiene 9. Adipic acid 81. Chloroform 152. Hydrochloric acid 10. Aldrin 82. Chloropyrifos 153. Hydrofluoric acid 11. Allyl alcohol 83. Chlorosulfonic acid 154. Hydrogen cyanide 12. Allyl chloride 84. Chromic acetate 155. Hydrogen sulfide 13. Aluminum sulfate 85. Chromic acid 156. Isoprene 14. Ammonia 86. Chromic sulfate 157. Isopropanolamine dodecylbenzenesulfonate 15. Ammonium acetate 87. Chromous chloride 158. Kelthane 16. Ammonium benzoate 88. Cobaltous bromide 159. Kepone 17. Ammonium bicarbonate 89. Cobaltous formate 160. Lead acetate 18. Ammonium bichromate 90. Cobaltous sulfamate 161. Lead arsenate 19. Ammonium bifluoride 91. Coumaphos 162. Lead chloride 20. Ammonium bisulfite 92. Cresol 163. Lead fluoborate 21. Ammonium carbamate 93. Crotonaldehyde 164. Lead fluorite 22. Ammonium carbonate 94. Cupric acetate 165. Lead iodide 23. Ammonium chloride 95. Cupric acetoarsenite 166. Lead nitrate 24. Ammonium chromate 96. Cupric chloride 167. Lead stearate 25. Ammonium citrate 97. Cupric nitrate 168. Lead sulfate 26. Ammonium fluoroborate 98. Cupric oxalate 169. Lead sulfide 27. Ammonium fluoride 99. Cupric sulfate 170. Lead thiocyanate 28. Ammonium hydroxide 100. Cupric sulfate ammoniated 171. Lindane 29. Ammonium oxalate 101. Cupric tartrate 172. Lithium chromate 30. Ammonium silicofluoride 102. Cyanogen chloride 173. Malathion 31. Ammonium sulfamate 103. Cyclohexane 174. Maleic acid 32. Ammonium sulfide 104. -

3.2.2 Table B: List of Dangerous Goods in Alphabetical Order the Following
Copyright © United Nations, 2008 3.2.2 Table B: List of dangerous goods in alphabetical order The following Table B is an alphabetical list of the substances and articles which are listed in the UN numerical order in Table A of 3.2.1. It does not form an integral part of ADN. It has been prepared, with all necessary care by the Secretariat of the United Nations Economic Commission for Europe, in order to facilitate the consultation of Annexes A and B, but it cannot be relied upon as a substitute for the careful study and observance of the actual provisions of those annexed Regulations which, in case of conflict, are deemed to be authoritative. NOTE 1: For the purpose of determining the alphabetical order the following information has been ignored, even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations "sec" and "tert"; and the letters "N" (nitrogen), "n" (normal), "o" (ortho) "m" (meta), "p" (para) and "N.O.S." (not otherwise specified). NOTE 2: The name of a substance or article in block capital letters indicates a proper shipping name (see 3.1.2). NOTE 3: The name of a substance or article in block capital letters followed by the word "see" indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs) (see 3.1.2.1). NOTE 4: An entry in lower case letters followed by the word "see" indicates that the entry is not a proper shipping name; it is a synonym. NOTE 5: Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name (see 3.1.2.1). -

Dielectric Constant Chart
Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ABS RESIN, LUMP 2.4-4.1 ABS RESIN, PELLET 1.5-2.5 ACENAPHTHENE 70 3 ACETAL 70 3.6 ACETAL BROMIDE 16.5 ACETAL DOXIME 68 3.4 ACETALDEHYDE 41 21.8 ACETAMIDE 68 4 ACETAMIDE 180 59 ACETAMIDE 41 ACETANILIDE 71 2.9 ACETIC ACID 68 6.2 ACETIC ACID (36 DEGREES F) 36 4.1 ACETIC ANHYDRIDE 66 21 ACETONE 77 20.7 ACETONE 127 17.7 ACETONE 32 1.0159 ACETONITRILE 70 37.5 ACETOPHENONE 75 17.3 ACETOXIME 24 3 ACETYL ACETONE 68 23.1 ACETYL BROMIDE 68 16.5 ACETYL CHLORIDE 68 15.8 ACETYLE ACETONE 68 25 ACETYLENE 32 1.0217 ACETYLMETHYL HEXYL KETONE 66 27.9 ACRYLIC RESIN 2.7 - 4.5 ACTEAL 21 3.6 ACTETAMIDE 4 AIR 1 AIR (DRY) 68 1.000536 ALCOHOL, INDUSTRIAL 16-31 ALKYD RESIN 3.5-5 ALLYL ALCOHOL 58 22 ALLYL BROMIDE 66 7 ALLYL CHLORIDE 68 8.2 ALLYL IODIDE 66 6.1 ALLYL ISOTHIOCYANATE 64 17.2 ALLYL RESIN (CAST) 3.6 - 4.5 ALUMINA 9.3-11.5 ALUMINA 4.5 ALUMINA CHINA 3.1-3.9 ALUMINUM BROMIDE 212 3.4 ALUMINUM FLUORIDE 2.2 ALUMINUM HYDROXIDE 2.2 ALUMINUM OLEATE 68 2.4 1 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ALUMINUM PHOSPHATE 6 ALUMINUM POWDER 1.6-1.8 AMBER 2.8-2.9 AMINOALKYD RESIN 3.9-4.2 AMMONIA -74 25 AMMONIA -30 22 AMMONIA 40 18.9 AMMONIA 69 16.5 AMMONIA (GAS?) 32 1.0072 AMMONIUM BROMIDE 7.2 AMMONIUM CHLORIDE 7 AMYL ACETATE 68 5 AMYL ALCOHOL -180 35.5 AMYL ALCOHOL 68 15.8 AMYL ALCOHOL 140 11.2 AMYL BENZOATE 68 5.1 AMYL BROMIDE 50 6.3 AMYL CHLORIDE 52 6.6 AMYL ETHER 60 3.1 AMYL FORMATE 66 5.7 AMYL IODIDE 62 6.9 AMYL NITRATE 62 9.1 AMYL THIOCYANATE 68 17.4 AMYLAMINE 72 4.6 AMYLENE 70 2 AMYLENE BROMIDE 58 5.6 AMYLENETETRARARBOXYLATE 66 4.4 AMYLMERCAPTAN 68 4.7 ANILINE 32 7.8 ANILINE 68 7.3 ANILINE 212 5.5 ANILINE FORMALDEHYDE RESIN 3.5 - 3.6 ANILINE RESIN 3.4-3.8 ANISALDEHYDE 68 15.8 ANISALDOXINE 145 9.2 ANISOLE 68 4.3 ANITMONY TRICHLORIDE 5.3 ANTIMONY PENTACHLORIDE 68 3.2 ANTIMONY TRIBROMIDE 212 20.9 ANTIMONY TRICHLORIDE 166 33 ANTIMONY TRICHLORIDE 5.3 ANTIMONY TRICODIDE 347 13.9 APATITE 7.4 2 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. -

6 NYCRR Part
6 NYCRR PART 597 HAZARDOUS SUBSTANCES IDENTIFICATION, RELEASE PROHIBITION, AND RELEASE REPORTING (Statutory authority: Environmental Conservation Law sections 1-0101, 3-0301, 3-0303, 17-0301, 17-0303, 17-0501, 17-1743, 37-0101 through 37-0107, and 40-0101 through 40-0121) TABLE OF CONTENTS 597.1 General. ....................................................................................................................2 597.2 Criteria for identifying a hazardous substance or acutely hazardous substance. .....5 597.3 List of hazardous substances. ...................................................................................6 597.4 Spills and Releases of hazardous substances. ..........................................................6 Table 1 Hazardous Substances – Sorted by Name ................................................................9 Table 2 Hazardous Substances – Sorted by CAS Number .................................................49 6 NYCRR Part 597 – Oct. 11, 2015 Page 1 of 88 597.1 597.1 General. (a) Purpose. The purpose of this Part is to: (1) set forth criteria for identifying a hazardous substance or acutely hazardous substance; (2) set forth a list of hazardous substances; (3) identify reportable quantities for the spill or release of hazardous substances; (4) prohibit the unauthorized release of hazardous substances; and (5) establish requirements for reporting of releases of hazardous substances. (b) Definitions. The following definitions apply to this Part: (1) Authorized means the possession of a valid license, permit, or certificate issued by an agency of the state of New York or the federal government, or an order issued by the Department or United States Environmental Protection Agency under applicable statutes, rules or regulations regarding the possession or release of hazardous substances or otherwise engaging in conduct which is exempt under applicable statutes, rules or regulations from the requirements of possessing such a license, permit, certificate or order. -
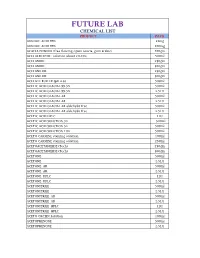
Qualikems Price List 2014-15
FUTURE LAB CHEMICAL LIST PRODUCT PACK ABSCISIC ACID 99% 25mg ABSCISIC ACID 99% 100mg ACACIA POWDER (free flowing) (gum acacia, gum arabic) 500gm ACETALDEHYDE solution about 20-30% 500ml ACETAMIDE 250gm ACETAMIDE 500gm ACETANILIDE 250gm ACETANILIDE 500gm ACETATE BUFFER (pH 4.6) 500ml ACETIC ACID GLACIAL 99.5% 500ml ACETIC ACID GLACIAL 99.5% 2.5Ltr ACETIC ACID GLACIAL AR 500ml ACETIC ACID GLACIAL AR 2.5Ltr ACETIC ACID GLACIAL AR aldehyde free 500ml ACETIC ACID GLACIAL AR aldehyde free 2.5Ltr ACETIC ACID HPLC 1Ltr ACETIC ACID SOLUTION 3% 500ml ACETIC ACID SOLUTION 5% 500ml ACETIC ACID SOLUTION 10% 500ml ACETO CARMINE staining solution 100ml ACETO CARMINE staining solution 250ml ACETOACETANILIDE (Tech) 250gm ACETOACETANILIDE (Tech) 500gm ACETONE 500ml ACETONE 2.5Ltr ACETONE AR 500ml ACETONE AR 2.5Ltr ACETONE HPLC 1Ltr ACETONE HPLC 2.5Ltr ACETONITRILE 500ml ACETONITRILE 2.5Ltr ACETONITRILE AR 500ml ACETONITRILE AR 2.5Ltr ACETONITRILE HPLC 1Ltr ACETONITRILE HPLC 2.5Ltr ACETO ORCEIN Solution 100ml ACETOPHENONE 500ml ACETOPHENONE 2.5Ltr ACETYLACETONE 250ml ACETYLACETONE 500ml ACETYLACETONE 2.5Ltr ACETYLACETONE AR 100ml ACETYLACETONE AR 500ml ACETYL BROMIDE 250ml ACETYL BROMIDE 500ml ACETYL CHLORIDE 500ml ACETYL CHLORIDE 2.5Ltr ACETYLCHOLINE CHLORIDE 25gm ACETYLCHOLINE CHLORIDE 100gm ACETYL SALICYLIC ACID 250gm ACETYL SALICYLIC ACID 500gm ACID ALCOHOL 125ml ACID ALCOHOL 500ml ACID MIXTURE (phosphoric/sulphuric) 125ml ACID MOLYBDATE solution 500ml ACRIDINE ORANGE (for Microscopical Staining) 10gm ACRIDINE ORANGE (for Microscopical Staining) 25gm -

Massachusetts Oil and Hazardous Material List
SUBPART P: MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST TABLE OF CONTENTS TABLE 1 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (ALPHABETICAL LISTING) TABLE 2 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (BY CAS NUMBER ORDER) NOTES: The Massachusetts Oil and Hazardous Materials List (MOHML) contains oils and hazardous materials subject to 310 CMR 40.0000 and their reportable quantities (RQs) and reportable concentrations (RCs). These values are referred to in the notification requirements (310 CMR 40.0300). This list is provided both alphabetically in Table 1 and by Chemical Abstracts Service Number (CAS Number) in Table 2. The CAS number is a unique number assigned to a substance. Both tables identify other lists on which a substance appears by using name source codes. These codes are as follows: Name Source 1 - The Department of Transportation (DOT) Hazardous Materials List (49 CFR Part 172.101 Hazardous Materials Table) Name Source 2 - The Resource Conservation and Recovery Act Appendix VIII List (40 CFR Part 261 - Appendix VIII Hazardous Constituents) Name Source 3 - The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Hazardous Substance and Waste Stream Lists (40 CFR Part 302 - Table 302.4) Name Source 4 - The Extremely Hazardous Substance List as mandated by Superfund Amendments and Reauthorization Act, Title III, Section 302 (40 CFR Part 355 Appendices A and B) Name Source 5 - DEP Allowable Ambient Limits (AALs) and Drinking Water Guidelines Name Source 6 - The Massachusetts Substance List (MSL)(105 CMR 670.000: “Right to Know” Appendix A) Name Source 7 - The Chemical Abstracts name, 9th collective period, 1972-1976 Name Source 8 - The EPA Right to Know list, Section 313 of the Emergency Planning and Community Right to Know Act of 1986 (40 CFR Part 372.65).