3.2.2 Table B: List of Dangerous Goods in Alphabetical Order the Following
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Antagonists for the Human Adenosine
UvA-DARE (Digital Academic Repository) Novel antagonists for the human adenosine A2A and A3 receptor via purine nitration: synthesis and biological evaluation of C2-substituted 6- trifluoromethylpurines and 1-deazapurines Koch, M. Publication date 2011 Document Version Final published version Link to publication Citation for published version (APA): Koch, M. (2011). Novel antagonists for the human adenosine A2A and A3 receptor via purine nitration: synthesis and biological evaluation of C2-substituted 6-trifluoromethylpurines and 1- deazapurines. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:05 Oct 2021 Novel antagonists for the human adenosine A Novel antagonists for the human adenosine Uitnodiging Novel antagonists for the voor het bijwonen van human adenosine de openbare verdediging van het proefschrift van A2A and A3 receptor via purine nitration Melle Koch op vrijdag 21 oktober 2011 om 14:00 uur in de Agnietenkapel van de Universiteit van Amsterdam Synthesis and biological evaluation Oudezijds Voorburgwal 231 of C2-substituted te Amsterdam 2A Na afloop van de promotie and A 6-trifluoromethylpurines zal hier tevens de receptie . -

Inventory Size (Ml Or G) 103220 Dimethyl Sulfate 77-78-1 500 Ml
Inventory Bottle Size Number Name CAS# (mL or g) Room # Location 103220 Dimethyl sulfate 77-78-1 500 ml 3222 A-1 Benzonitrile 100-47-0 100ml 3222 A-1 Tin(IV)chloride 1.0 M in DCM 7676-78-8 100ml 3222 A-1 103713 Acetic Anhydride 108-24-7 500ml 3222 A2 103714 Sulfuric acid, fuming 9014-95-7 500g 3222 A2 103723 Phosphorus tribromide 7789-60-8 100g 3222 A2 103724 Trifluoroacetic acid 76-05-1 100g 3222 A2 101342 Succinyl chloride 543-20-4 3222 A2 100069 Chloroacetyl chloride 79-04-9 100ml 3222 A2 10002 Chloroacetyl chloride 79-04-9 100ml 3222 A2 101134 Acetyl chloride 75-36-5 500g 3222 A2 103721 Ethyl chlorooxoacetate 4755-77-5 100g 3222 A2 100423 Titanium(IV) chloride solution 7550-45-0 100ml 3222 A2 103877 Acetic Anhydride 108-24-7 1L 3222 A3 103874 Polyphosphoric acid 8017-16-1 1kg 3222 A3 103695 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103694 Chlorosulfonic acid 7790-94-5 100g 3222 A3 103880 Methanesulfonic acid 75-75-2 500ml 3222 A3 103883 Oxalyl chloride 79-37-8 100ml 3222 A3 103889 Thiodiglycolic acid 123-93-3 500g 3222 A3 103888 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 103886 Tetrafluoroboric acid 50% 16872-11-0 1L 3222 A3 102969 sulfuric acid 7664-93-9 500 mL 2428 A7 102970 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102971 hydrochloric acid (37%) 7647-01-0 500 mL 2428 A7 102973 formic acid (88%) 64-18-6 500 mL 2428 A7 102974 hydrofloric acid (49%) 7664-39-3 500 mL 2428 A7 103320 Ammonium Hydroxide conc. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
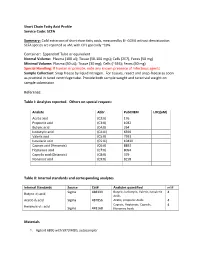
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Medicine with Deeper, More Biochemical Conception Of
Edinburgh Medical Journal May 1951 APPLICATIONS of chemical defence research IN MEDICINET? By Professor R. A. PETERS to be invited It is a great responsibility, as well as a great honour, to Cameron lecturers give this lecture ; and I expect that with other I this ancient seat of share a sense of awe upon standing here in is learning. For me it is an awe tinged with much feeling (it possible that this is partly because some forebears of my mother came from near to recent of the this ; it is in part due the perusal city) perhaps " address here Froude in on the Times of John Knox, given by 1865 " " entitled Influence of the Reformation upon the Scottish Character ; this address drives home to the Sassenach the sterner stuff of which all of us in the the Scots are made ; but I really think it is more that as south, even if we come as I do from a Medical School as ancient the Hospital of St Bartholomew, know full well that Edinburgh stands high indeed in the world of Medical Schools as Scotland itself stands in the world of intellect. Elsewhere I have discussed fully the historical background of our for me back researches upon Chemical Defence substances, which go to World War I.23 Again in my Dixon lecture,24 I have indicated some of the interest for pharmacology. The outlook for medicine has not the been reviewed so far. Therefore I propose to-day to discuss of British influence upon the future of therapeutics of the discovery anti-lewisite and of some of the other researches upon war gas but substances ; and to consider not only the leads towards therapy, the possible newer trends given to medicine itself. -

United States Patent (19) 11 Patent Number: 5,253,711 Mondshine (45) Date of Patent: Oct
USOO525371 1A United States Patent (19) 11 Patent Number: 5,253,711 Mondshine (45) Date of Patent: Oct. 19, 1993 54) PROCESS FOR DECOMPOSING 2,268,215 12/1941 Kerr ...................................... 127/33 POLYSACCHARDES IN ALKALINE 3,167,510 /1965 Alter ..... sa as A8 a X8 a P. 252/8.551 3,655,644 4/1972 Durand ........................... 106/21 X AQUEOUS SYSTEMS 3,935,187 1/1976 Speakman ........................... 536/102 75 Inventor: Thomas C. Mondshine, Houston, 4,202,795 5/1980 Burnham et al. ............... 166/308 X Tex. 4,552,668 11/1985 Brown et al. ................... 166/300X Lachenal et al. ..................... 162/25 Assignee: Texas United Chemical Corp., 4,787,959 11/1988 (73) Primary Examiner-George A. Suchfield Houston, Tex. Attorney, Agent, or Firm-Roy F. House 21 Appl. No.: 844,167 57 ABSTRACT 22 Filed: Mar. 2, 1992 Alkaline earth metal or transition metal peroxides are (51) int. Cli.............................................. E21B 43/26 used as a delayed breaker in alkaline aqueous fluids 52) U.S. C. .................................... 166/300; 166/308; containing a water soluble hydrophilic polysaccharide 252/8.551; 252/326 polymer hydrated therein. The peroxide is activated by (58) Field of Search ............................... 166/300, 308; increasing the temperature of the fluid. The invention is 252/8.551, 326,358; 536/41, 80, 88 particularly useful for the delayed break of hydraulic 56) References Cited fracturing fluids containing hydroxypropyl guar poly c. U.S. PATENT DOCUMENTS i,953,398 4/1934 Eskew ................................... 536/41 10 Claims, No Drawings 5,253,711 1. 2 G. W. Hawkins, and H. D. Brannon, Feb. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Decision-Making in Chemical Warfare Agent (CWA) Response There Is a Lot of Fear Associated with Chemical Will Act Accordingly
Application Note: 102 Decision-Making in Chemical Warfare Agent (CWA) Response There is a lot of fear associated with Chemical will act accordingly. If the first responders Warfare Agents (CWAs). The misnomer over-react and immediately jump into full “Nerve Gas” quickly brings horrible images to encapsulation protection it could panic the the minds of many civilians. But if we lay aside public and cause unnecessary worry and the politics and fear, CWA detection should even injury. treated like other gas/vapor detection challenges. It should be a collaborative Over Protection Can Be Dangerous process encompassing physical clues, threat to the Responder scenario, biological clues, and a variety of Heat stress is the number one injury in sensing technologies. No one clue or HazMat response and immediately jumping technology is always correct. Experience and into full Level A encapsulation is a good way the use of multiple clues and technologies are of overheating oneself. Level A the keys to successful CWA response. encapsulation also makes one much more Understanding what the clues are and how to susceptible to slip, trip and fall injuries. layer them to make a decision is critical to Finally, over protection makes it harder to get successful CWA response. things done. When properly used, detection allows responders to respond at lower levels Why is Gas Detection Important? of Personal Protective Equipment (PPE) to Responders cannot rely on their senses for provide the highest levels of safety to decision-making. Without effectively knowing themselves and to the community that they how to use detection techniques responders protect. -

Status of Industry Efforts to Replace Halon Fire Extinguishing Agents
STATUS OF INDUSTRY EFFORTS TO REPLACE HALON FIRE EXTINGUISHING AGENTS Robert T. Wickham, P.E. March 16, 2002 WICKHAM ASSOCIATES NOTICE This document is disseminated under the sponsorship of the U.S. Environmental Protection Agency in the interest of information exchange. The views expressed in this report are those of the author and do not necessarily reflect those of the US Environmental Protection Agency. The US Environmental Protection Agency does not assume liability for the contents or use thereof and further the Agency does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. The following commercial products (requiring a trademark designation ® or ™) are mentioned in this report. Due to the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Ansul FE-25 Argonite FE-36 Argotec FM-200 CEA-410 Halotron I CEA-614 Inergen CEA-308 NAF P-IV Envirogel NAF S-III FE-13 NN100 FE-227 Triodide FE-241 There are no restrictions on copying or distribution of this document. Additional copies of this report are available from ….. Wickham Associates 9 Winding Brook Drive Stratham, New Hampshire 03885 USA Tel: +603-772-3229 Fax: +603-772-7305 email: [email protected] The report is available at http://home.attbi.com/~wickham/downloads.htm for downloading in Adobe Acrobat portable document format (pdf). Preface This report is not intended to be a market study, but instead a snapshot of the progress industry and the government are making in employing non ozone depleting alternatives to halons in the fire protection sector. -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -

' ' T UNITED' STATES PATENT 'V OFFICE
PatentedUNITED’ Apr.v22,_,>1947'-" STATES > PATENT' ‘ 'v TOFFICE ‘2,419,488 " PRODUCTION OF MoNocnLoRo DERIVA- ‘ ~ ' 'rrvns 0F UNSATUBATED NITRILES - ' Harris A. Dutcher, Borg'cr', ‘_Tex_., asslgnor 'to_ 4 Phillips Petroleum Company,.a corporation of ‘.Delaware> f . ' ' > v No Drawing. Application June 5,1944, I, > Serial No. 538,880 ‘ , ' 11 Claims. (01. zed-464$ , . 2 . The present invention relates to the production - Heretofore, a-chloroacrylonitrile has been pro of chlorine derivatives of unsaturated nitriles by ' duced as a by-product oi.‘ the direct substitutive the reaction of acetylene or acetylenic hydrocar- chlorination of acrylonitrile in the vapor phase bons with cyanogen chloride. More particularly over active carbon at a temperature between ap the invention relates to the production of 3- 5 proximately 200° and approximately 550° C. chloroacrylonitrile - ‘ . (Long, U. S. Patent No. 2,231,363). The process (Z-chloroviny] cyanide,‘ C1__CH___CH_CN) yields 2-chloroacrylonitrile as the principal prod uct. vNo other methods for the production of 3 rgldsl?ltlgfesmigoilxgr196ii?ggtggesagty‘gfgtg; chloroacrylonitrile or other monochloro deriva acetylenic hydrocarbons and cyanogen chloride 10 tives of unsaturatednitriles are known. ‘ ' ’ I have found that acetylene and acetylenic hy is 2281322232”; ‘13355;:ffgléetggesggtdilrgggi? . drocarbons may be reacted with cyanogen halides . I . v to produce.monohalogen-substituted unsaturated monochloro derivatives of unsaturated mtnles, . 1 such as 3-chloroacrylonitrile by there'action of- 1 mtr?es' The reactlon 1.5 facihtated ‘by certain ’ - » >15 catalysts which are referred to more speci?cally acetylene and cyanogen chloride. , hereinafter _ provideAnother a categlyst ob'ect (1201‘f thpfoggtiiirgtthevggg?lo?rg- e ‘n ' ' ‘ o Cyanogen' ‘ihlmd‘?. 157a readny.condens1ble.